
Am Fam Physician. 2025;111(1):12-14
An audio version of this AFP editorial is available on the AAFP audio app.
Author disclosure: Dr. Campos-Outcalt is a paid consultant to ACIP.
The US Food and Drug Administration (FDA) in June 2024 approved the use of a 21-valent pneumococcal conjugate vaccine (PCV21; Capvaxive) for adults 18 years and older to prevent invasive pneumococcal diseases such as pneumonia, meningitis, and sepsis. At its June meeting, the Advisory Committee on Immunization Practices (ACIP) recommended PCV21 as an option for vaccinating adults 18 years and older.1 Vaccination against pneumococcal disease was, until recently, recommended for all adults 65 years and older and for adults 19 to 64 years of age who have an immunocompromising condition that increases the risk of pneumococcal infection or a chronic medical condition that increases the risk of serious pneumococcal disease if infected (Table 1).1 In October 2024, the ACIP voted to reduce the age of routine PCV to 50 years and to continue to recommend PCV for adults younger than 50 years with risk conditions.
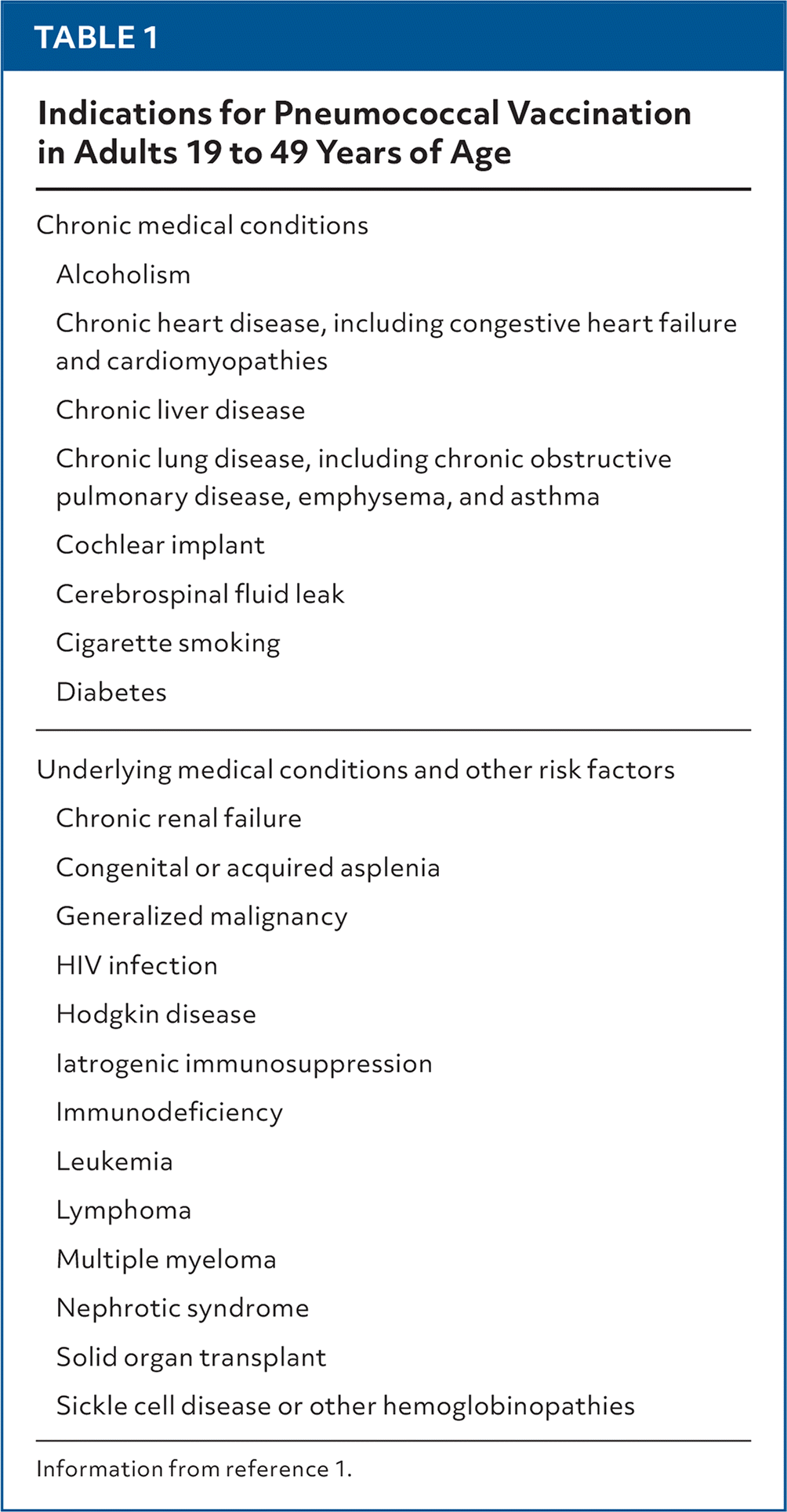
| Chronic medical conditions |
| Alcoholism |
| Chronic heart disease, including congestive heart failure and cardiomyopathies |
| Chronic liver disease |
| Chronic lung disease, including chronic obstructive pulmonary disease, emphysema, and asthma |
| Cochlear implant |
| Cerebrospinal fluid leak |
| Cigarette smoking |
| Diabetes |
| Underlying medical conditions and other risk factors |
| Chronic renal failure |
| Congenital or acquired asplenia |
| Generalized malignancy |
| HIV infection |
| Hodgkin disease |
| Iatrogenic immunosuppression |
| Immunodeficiency |
| Leukemia |
| Lymphoma |
| Multiple myeloma |
| Nephrotic syndrome |
| Solid organ transplant |
| Sickle cell disease or other hemoglobinopathies |
There are now three options for vaccinating adults against pneumococcal disease: PCV15 followed by 23-valent pneumococcal polysaccharide vaccine (PPSV23); PCV20; and PCV21. The composition of each vaccine is described in Figure 1.1 PCV20 contains all of the serotypes in PCV15 plus five additional serotypes. PCV21 does not include antigens for 10 serotypes contained in PCV20 but adds antigens for 11 other serotypes not contained in PCV15 or PCV20. However, PCV21 does not contain serotype 4, which has implications for certain populations that recently have had high rates (30% or greater) of invasive pneumococcal disease due to serotype 4, particularly in Alaska, Colorado, the Navajo Nation, New Mexico, and Oregon.1 Those at risk of serotype 4 pneumococcal disease are typically adults younger than 65 years with a risk condition and a history of substance abuse or homelessness.
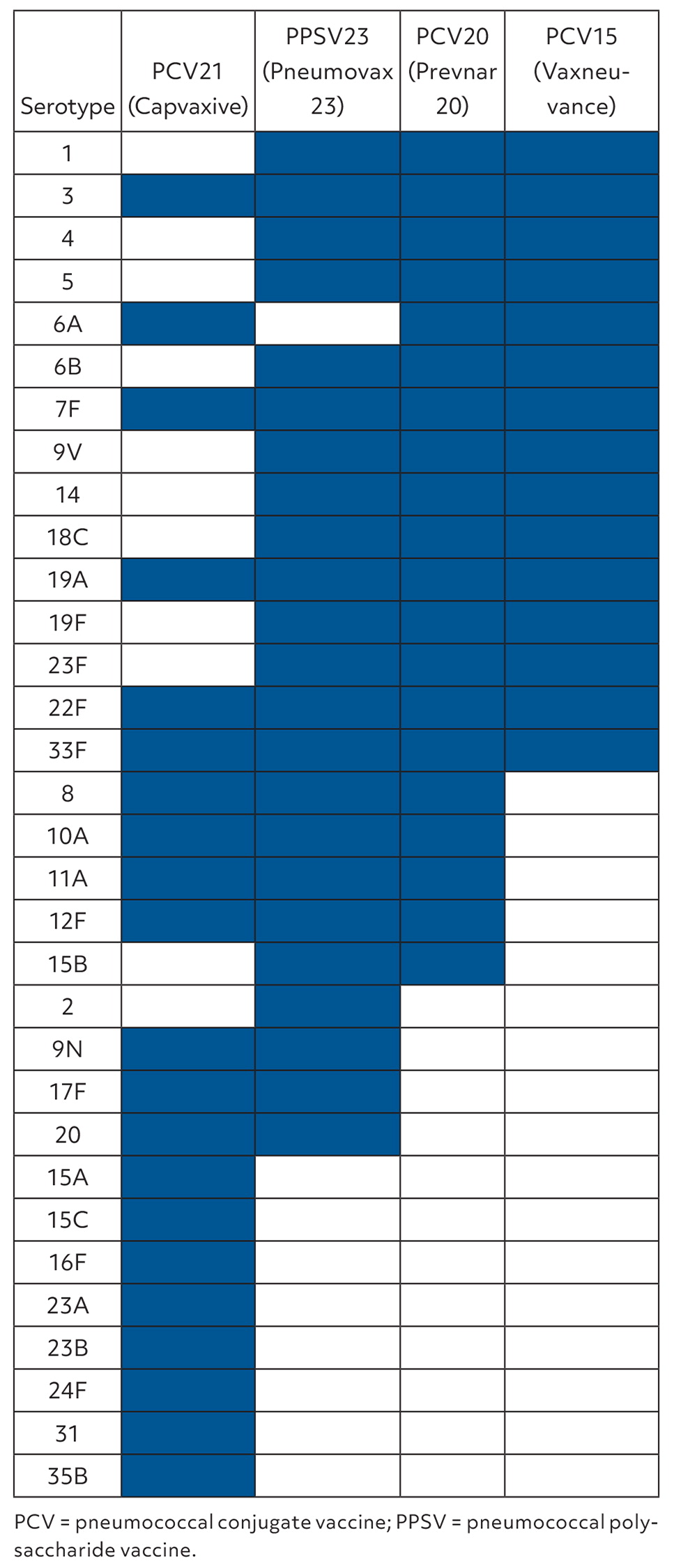
The vaccines available to protect against pneumococcal disease have changed over time. PPSV23 was first recommended in 1984 for older adults and those with chronic medical conditions. PCV7 was first recommended for children in 2000, then replaced by PCV13 in 2010. In addition to PPSV23, PCV13 was recommended for adults with immunocompromising conditions in 2012 and for all older adults in 2014. The two options of PCV15 followed by PPSV23 or PCV20 alone were recommended in late 2021.2 PCV21 has now been added as a third option.
This sequence of different PCV products and changing recommendations has led to a number of possibilities for patients presenting with varying pneumococcal vaccination histories. This generates questions about what vaccine is recommended for PCV naive patients, those who are incompletely vaccinated (PPSV23, PCV7, or PCV13 alone), and those who previously received lower valent products, even if followed by PPSV23. Table 2 summarizes a few of the possible scenarios, but the most recent Centers for Disease Control and Prevention recommendations should be consulted for other scenarios.1 A key point to remember is that if an adult has received PCV20 or PCV21, no further PCV vaccinations are recommended. The need for boosters will be determined with time.
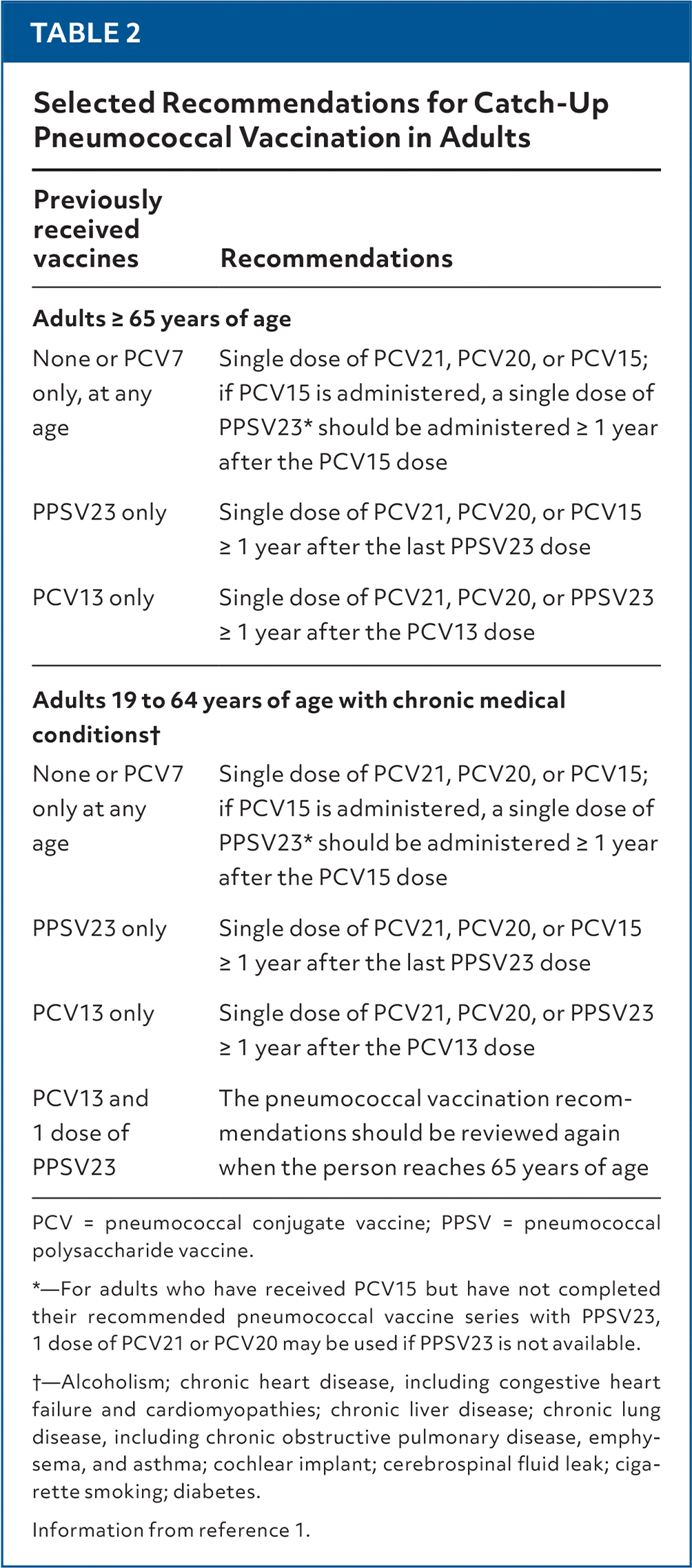
| Previously received vaccines | Recommendations |
|---|---|
| Adults ≥ 65 years of age | |
| None or PCV7 only, at any age | Single dose of PCV21, PCV20, or PCV15; if PCV15 is administered, a single dose of PPSV23* should be administered ≥ 1 year after the PCV15 dose |
| PPSV23 only | Single dose of PCV21, PCV20, or PCV15 ≥ 1 year after the last PPSV23 dose |
| PCV13 only | Single dose of PCV21, PCV20, or PPSV23 ≥ 1 year after the PCV13 dose |
| Adults 19 to 64 years of age with chronic medical conditions† | |
| None or PCV7 only at any age | Single dose of PCV21, PCV20, or PCV15; if PCV15 is administered, a single dose of PPSV23* should be administered ≥ 1 year after the PCV15 dose |
| PPSV23 only | Single dose of PCV21, PCV20, or PCV15 ≥ 1 year after the last PPSV23 dose |
| PCV13 only | Single dose of PCV21, PCV20, or PPSV23 ≥ 1 year after the PCV13 dose |
| PCV13 and 1 dose of PPSV23 | The pneumococcal vaccination recommendations should be reviewed again when the person reaches 65 years of age |
If you think things are complicated now, it is going to get worse. There are three more PCV products in the pipeline: two 24-valent and one 31-valent. However, it is anticipated that market forces may nudge the PCV15-PPSV23 combination out of common use because the PCV20 and PCV21 options are simpler.
