
Am Fam Physician. 2025;111(2):110-112
Author disclosure: No relevant financial relationships.
The US Preventive Services Task Force (USPSTF) recently celebrated its 40th anniversary. The goal of the USPSTF is to make evidence-based recommendations regarding screening and prevention that improve health and prolong life; cost has not traditionally been considered, only potential benefits and harms. The USPSTF has been lauded as a model for guideline development due to its rigorous evidence-based processes.1 Each of the 88 recommendations is based on an independent systematic review and an assessment and discussion of outcomes and quality of evidence by USPSTF members, with input from external reviewers and the public. Each final recommendation statement must then be ratified by a vote.
For some preventive services, the USPSTF determines that a recommendation cannot be made due to insufficient evidence. Most graded recommendations are based on direct evidence from randomized controlled trials (RCTs) comparing the benefits and harms of a screening test or preventive service with no intervention or placebo. Examples include all recommended cancer screening tests, aspirin for primary prevention, and medications to reduce the risk of breast cancer. For some conditions, such as HIV infection, no RCTs of screening have evaluated patient-oriented outcomes.2 In this case, the USPSTF has recommended screening based on a causal chain: the screening tests are accurate, and treatments reduce the risk of death, AIDS-related illness, and transmission.
However, some recent recommendations have raised concerns about the consistency of the USPSTF's processes and how they are applied (Table 13–12). Potential problems include an overreliance on modeling projections, recommendations that have changed in the apparent absence of new evidence, and recommendations that appear to be inconsistent with the best available evidence from clinical trials.
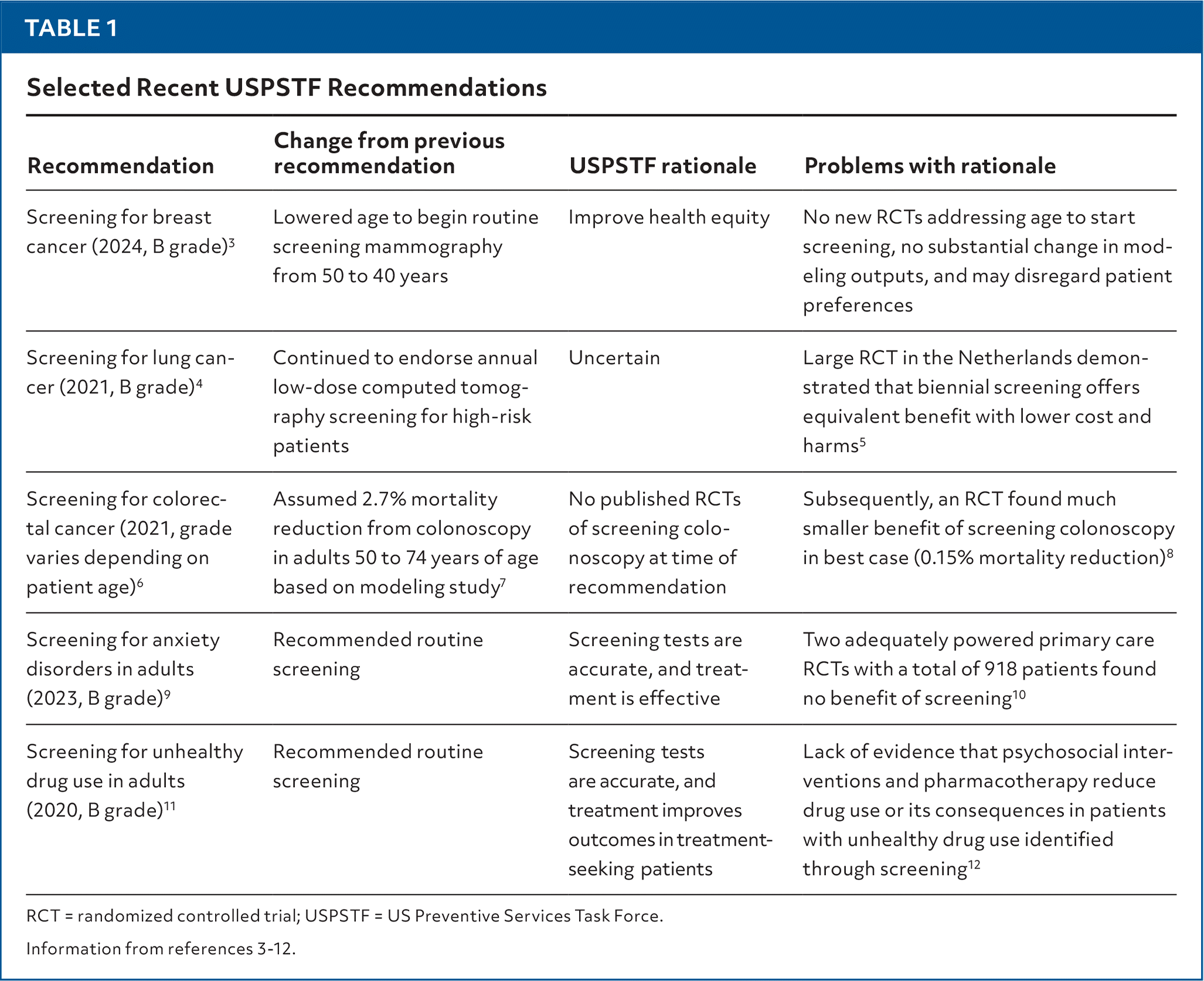
| Recommendation | Change from previous recommendation | USPSTF rationale | Problems with rationale |
|---|---|---|---|
| Screening for breast cancer (2024, B grade)3 | Lowered age to begin routine screening mammography from 50 to 40 years | Improve health equity | No new RCTs addressing age to start screening, no substantial change in modeling outputs, and may disregard patient preferences |
| Screening for lung cancer (2021, B grade)4 | Continued to endorse annual low-dose computed tomography screening for high-risk patients | Uncertain | Large RCT in the Netherlands demonstrated that biennial screening offers equivalent benefit with lower cost and harms5 |
| Screening for colorectal cancer (2021, grade varies depending on patient age)6 | Assumed 2.7% mortality reduction from colonoscopy in adults 50 to 74 years of age based on modeling study7 | No published RCTs of screening colonoscopy at time of recommendation | Subsequently, an RCT found much smaller benefit of screening colonoscopy in best case (0.15% mortality reduction)8 |
| Screening for anxiety disorders in adults (2023, B grade)9 | Recommended routine screening | Screening tests are accurate, and treatment is effective | Two adequately powered primary care RCTs with a total of 918 patients found no benefit of screening10 |
| Screening for unhealthy drug use in adults (2020, B grade)11 | Recommended routine screening | Screening tests are accurate, and treatment improves outcomes in treatment-seeking patients | Lack of evidence that psychosocial interventions and pharmacotherapy reduce drug use or its consequences in patients with unhealthy drug use identified through screening12 |
Modeling has an important role in evaluating benefits and harms, different ages to begin and stop screening, and different screening intervals in the absence of RCTs.13 However, it has become clear that modeling has limitations in accurately projecting health outcomes from a screening program. For example, the USPSTF modeling study for colorectal cancer screening estimates that for every 1,000 people 50 to 74 years of age undergoing colonoscopy every 10 years, 27 colorectal cancer deaths are averted (a 2.7% reduction), at the cost of 14 serious complications.7 But a subsequently published RCT comparing colonoscopy with no screening in patients 55 to 64 years of age found that even with the most optimistic analysis, the reduction in mortality was only 0.15% after 10 years.8
Even with a longer timeline, it is difficult to imagine that the mortality reduction in the trial participants would approach 2.7%. The USPSTF acknowledges the limitations of modeling, and it is hoped that discrepancies like this will be studied to improve future models.13
Regarding breast cancer screening for women 40 to 49 years of age, the USPSTF recently updated the recommendation from shared decision-making based on individual risk and values to screening for all women beginning at age 40.3 This change occurred despite no new RCTs on the benefits and harms of different starting ages and no substantial change in modeling outputs.14 In addition, it is in the context that up to 4 in 10 women in the United States in this age group prefer to delay screening after being informed of the benefits and harms, and that experiencing a false-positive mammogram result is associated with a reduction in the likelihood that a patient will return for subsequent mammograms.15,16
A stated goal of the USPSTF is a desire to improve equity, and although Black women already have higher screening rates than White women or women of Asian descent, they also have higher mortality rates for breast cancer.17 In this case, enhancing equity may come with a higher burden or harm for some women, with uncertain additional benefit. Such trade-offs should be discussed more explicitly.
In some cases, updated recommendations do not align with data from existing or newly published RCTs. For example, a large study on lung cancer screening in the Netherlands found outcomes that were equivalent to or better than those of the comparable trial in the United States. The study was also able to gradually lengthen the testing interval from 1 to 2.5 years, reducing cost and harm without compromising benefit.5 Based on updated modeling, the USPSTF statement a year later continued to recommend annual screening.4 We suggest that, in the face of new evidence and the opportunity to reduce harms, burden, and cost by safely lengthening intervals, evidence from both new trials and modeling be used. For example, the recommendation could explicitly incorporate the new trial evidence and state that intervals can safely be lengthened over time.
Another example is the 2023 recommendation to screen for anxiety disorders in primary care settings.9 The recommendation rests on the indirect evidence that screening tests are accurate and treatment is effective. However, two adequately powered primary care RCTs with a total of 918 patients found no benefit to screening.10 In this case, direct evidence was outweighed by indirect evidence, a break with previous USPSTF methods. Potential harms of anxiety screening include increased time and documentation burdens and adverse effects of unnecessary medications.18
Similarly, the 2020 recommendation to routinely screen adults for unhealthy drug use relied on a causal chain showing that screening questions identified individuals with unhealthy drug use and that interventions in treatment-seeking populations improved health outcomes.11 However, there are important differences between patients with substance use disorders identified through screening vs those seeking treatment.19 Notably, the USPSTF's evidence report concluded that although pharmacotherapy and psychosocial interventions effectively improve drug use outcomes, evidence of effectiveness is primarily derived from trials conducted in treatment-seeking populations.12 A cluster RCT published after the recommendation statement was released found that screening for opioid use disorder in diverse primary care settings in the United States did not increase the rate of diagnosis.20 We hope that this new evidence is used to update the recommendation to better reflect the lack of evidence in screened populations.
Primary care physicians have come to trust and rely on USPSTF recommendations in clinical practice, and its A and B grade assessments have been linked to mandatory private insurance coverage without cost-sharing since 2010.21 To justify the extra time and effort associated with implementing new or expanded screening recommendations, clinicians must have confidence in the reliability of USPSTF assessments regardless of the task force's membership at any point in time. We hope that this commentary sheds light on why the recommendations of the American Academy of Family Physicians occasionally differ from those of the USPSTF22 and encourages current and future task force members to continue to improve their methods for assessing the benefits and harms of clinical preventive services.
Sir Muir Gray, a preventive medicine expert in the United Kingdom, stated that “All screening programmes do harm: some do good as well, and, of these, some do more good than harm at reasonable cost.” The first task of any public health service is to identify beneficial programs by appraising the evidence.23
Editor’s Note: Dr. Ebell was a member of the USPSTF from 2012 to 2015 and a consultant to the USPSTF from 2016 to 2020. Dr. Lin was a medical officer at the Agency for Healthcare Research and Quality supporting the USPSTF from 2006 to 2010. Both are members of the AAFP Science Advisory Panel and deputy editors for AFP.
