
Am Fam Physician. 2025;111(3):202-204
Author disclosure: The authors are salaried employees of Healthmap Solutions, a population health management company that helps guide health plans and groups of clinicians in improving care for patients with kidney disease.
In March 2024, Kidney Disease: Improving Global Outcomes (KDIGO) published updated guidelines on the evaluation and management of chronic kidney disease (CKD).1 A comprehensive review of screening for and evaluation and management of CKD and its complications was published previously in American Family Physician.2 This editorial highlights the KDIGO updates related to new medications that can delay CKD progression and recommendations for assessment of atherosclerotic cardiovascular disease (ASCVD) risk in patients with CKD.
LIFESTYLE MODIFICATION
Increasing physical activity
Weight management
Cessation of tobacco use
Consumption of a whole-food, plant-based diet that minimizes intake of animal-based and ultra-processed foods
When available, referral to a renal dietitian is beneficial for patients with CKD because of their complex dietary needs. These include adjustment in intake of sodium, potassium, phosphorus, protein, and fluids, while also accounting for comorbidities.
RENIN-ANGIOTENSIN SYSTEM INHIBITORS
Renin-angiotensin system inhibitors, which include angiotensin-converting enzyme inhibitors and angiotensin receptor blockers, have a well-established history of reducing all-cause mortality and providing renal and cardioprotective benefits in adults who have CKD with or without diabetes.4,5 The KDIGO guideline recommends titrating the dosage of renin-angiotensin system inhibitors to achieve a systolic blood pressure less than 120 mm Hg in patients with high blood pressure and CKD. A higher goal is reasonable for patients with frailty, at risk for falls, or with limiting factors such as symptomatic postural hypotension.
For patients with nonemergent hyperkalemia, the KDIGO guideline has shifted away from recommending reflexive discontinuation of renin-angiotensin system inhibitors. Instead, management suggestions include reviewing modifiable factors such as dietary potassium intake, eliminating medications that increase serum potassium, and prescribing potassium-lowering medications such as diuretics when appropriate. The National Kidney Foundation provides a patient-friendly overview of high- and low-potassium foods in English and Spanish. Referral to a nephrologist may also be appropriate.
Clinicians can initiate newer potassium-binding agents such as patiromer (Veltassa) or sodium zirconium cyclosilicate (Lokelma). These are well-tolerated and safe for long-term use compared with earlier agents.
SODIUM-GLUCOSE COTRANSPORTER-2 INHIBITORS
Sodium-glucose cotransporter-2 (SGLT-2) inhibitors reduce risk of acute kidney injury and progression to kidney failure and provide cardioprotective benefits for adults with CKD and type 2 diabetes.1 A significant update in the 2024 KDIGO guideline is that these benefits have also been seen in adults with CKD without diabetes who have albuminuria (defined as a 200 mg/g [20 mg/mmol] or greater albumin to creatinine ratio) or a diagnosis of heart failure.1,6
Similar to renin-angiotensin system inhibitors, SGLT-2 inhibitors may cause a brief, reversible decrease in estimated glomerular filtration rate (eGFR) at initiation, which typically ranges between 3 and 5 mL/min/1.73 m2.7 Depending on baseline eGFR, the decrease may represent a 10% or greater decline. This is an expected hemodynamic effect of SGLT-2 inhibition resulting in preservation of kidney function over time and generally should not warrant a change in CKD monitoring or therapy cessation.
Potential adverse effects of SGLT-2 inhibitors include vulvovaginal candidiasis, perineal infection, volume depletion, and ketoacidosis. These medications should be withheld 3 days before scheduled surgeries (4 days for ertugliflozin [Steglatro]).8
STATINS
Although cardiovascular disease is the primary cause of death in patients with CKD regardless of stage, older risk prediction models underestimated ASCVD risk in patients with CKD. Another significant update in the KDIGO guideline is the recommendation for use of ASCVD risk estimators that either have been validated in cohorts of individuals with CKD or that incorporate eGFR and albuminuria.1 Such tools include the Systematic Coronary Risk Evaluation CKD patch and the Predicting Risk of Cardiovascular Disease Events calculator.
The KDIGO guideline emphasizes use of statins in most patients with CKD (Figure 1).1 Statins that do not require renal dosing adjustments include the high-intensity statin atorvastatin and moderate-intensity statins pravastatin and simvastatin. Rosuvastatin is a high-intensity statin requiring dosing adjustment, with a maximum recommended dose of 10 mg/day if the creatinine clearance is less than 30 mL/min/1.73 m2.
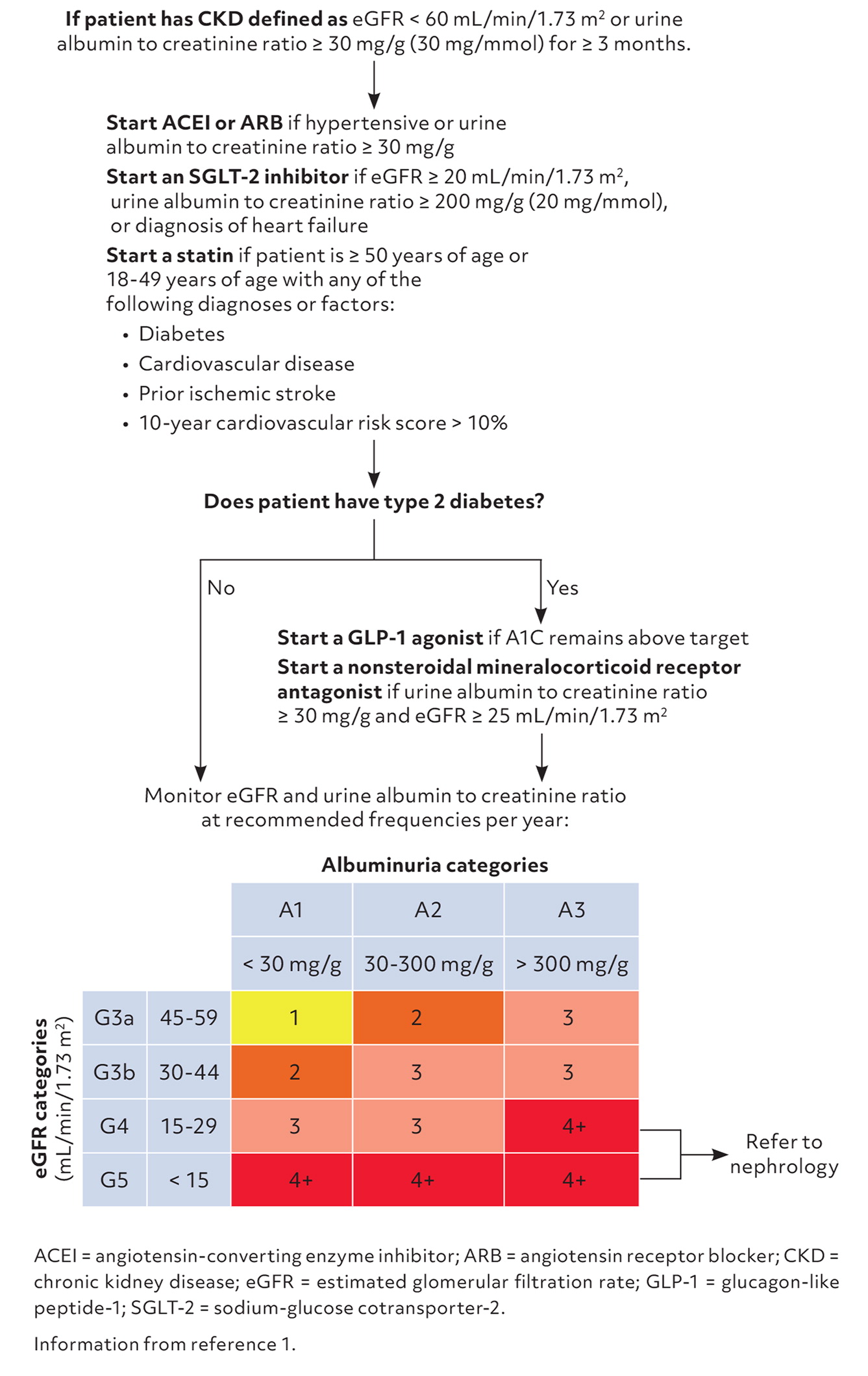
GLUCAGON-LIKE PEPTIDE-1 AGONISTS
In addition to improving glycemic control and promoting weight loss, glucagon-like peptide-1 (GLP-1) agonists have been shown to slow progression of kidney failure and reduce risk of cardiovascular-related and all-cause mortality in adults with CKD and type 2 diabetes.9,10 GLP-1 agonists that do not require renal dosing adjustments and are associated with cardiovascular benefits include dulaglutide (Trulicity), liraglutide (Victoza), and semaglutide (Ozempic).
KDIGO recommends measurement of A1C levels to monitor glycemic control rather than glycated albumin or fructosamine.11 An individualized, varying A1C goal of less than 8.0% is appropriate based on patient factors such as life expectancy, CKD severity, medical comorbidities, macrovascular complications, hypoglycemia awareness, and propensity of medications to cause hypoglycemia. GLP-1 agonist dosing can be titrated every 4 weeks to achieve glycemic goals.
NONSTEROIDAL MINERALOCORTICOID RECEPTOR ANTAGONISTS
Finerenone (Kerendia) is the only nonsteroidal mineralocorticoid receptor antagonist available in the United States. It slows CKD progression and reduces cardiovascular risk, specifically hospitalizations for heart failure, in adults with CKD and type 2 diabetes.12 Given its antagonism on the mineralocorticoid receptor, physicians should be aware of the risk of hyperkalemia with this drug. Finerenone should be withheld if the serum potassium level exceeds 5.5 mEq/L (5.5 mmol/L). Due to its short, 2- to 3-hour half-life, finerenone can be safely reinitiated after the serum potassium level returns to 5.5 mEq/L or less.13
