
Am Fam Physician. 2025;111(4):311-312
Author disclosure: No relevant financial relationships.
DETAILS FOR THIS REVIEW
Study Population: 43 randomized controlled trials that included 11,419 men 40 years and older with sexual dysfunction; sexual dysfunction was defined as acquired symptoms of decreased libido or erectile dysfunction1
Efficacy End Points: Erectile function, sexual quality of life
Harm End Points: Cardiovascular mortality, prostate-related events, lower urinary tract symptoms, and treatment withdrawal due to adverse events
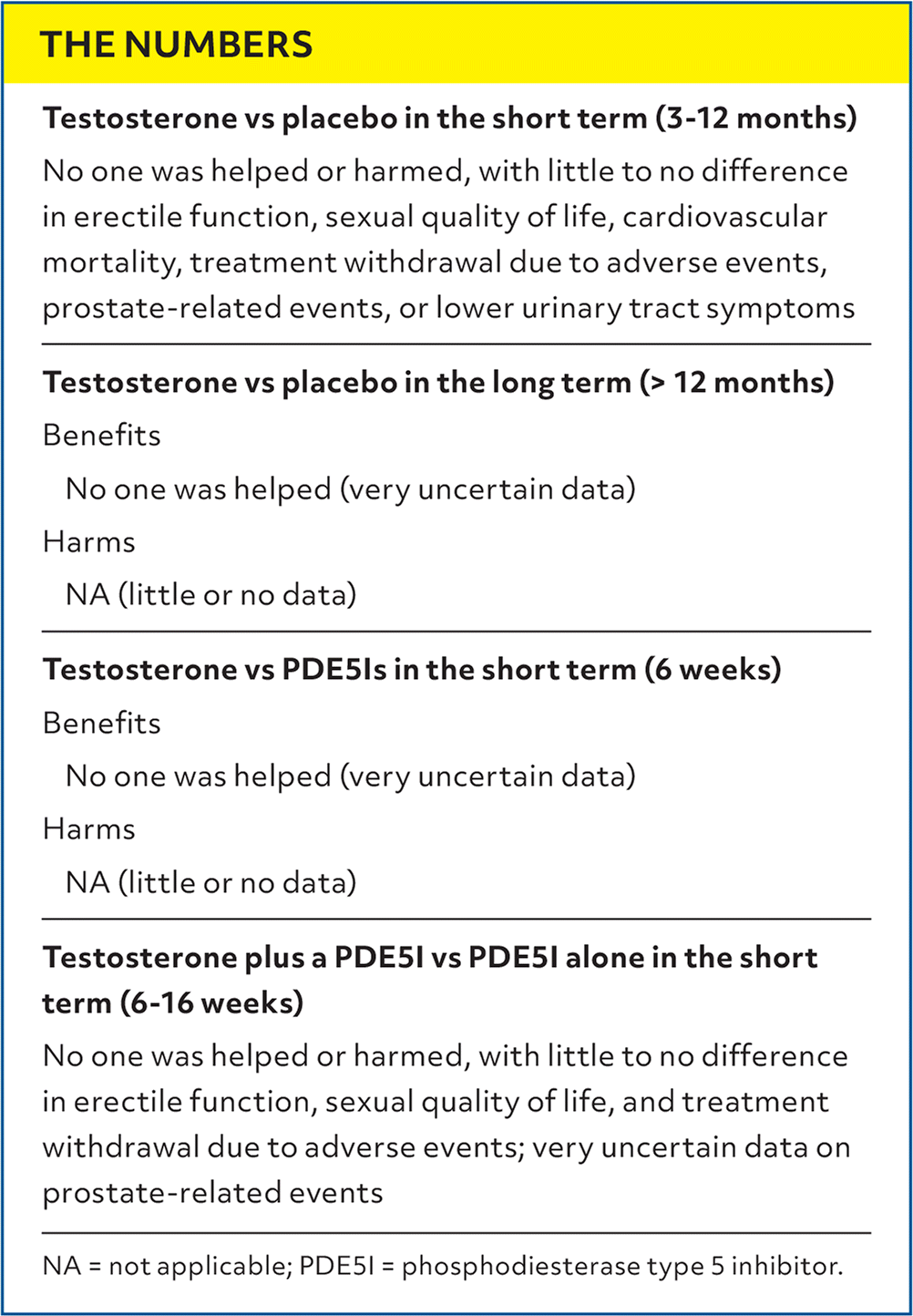
| Testosterone vs placebo in the short term (3–12 months) |
| No one was helped or harmed, with little to no difference in erectile function, sexual quality of life, cardiovascular mortality, treatment withdrawal due to adverse events, prostate-related events, or lower urinary tract symptoms |
| Testosterone vs placebo in the long term (> 12 months) |
| Benefits |
| No one was helped (very uncertain data) |
| Harms |
| NA (little or no data) |
| Testosterone vs PDE5Is in the short term (6 weeks) |
| Benefits |
| No one was helped (very uncertain data) |
| Harms |
| NA (little or no data) |
| Testosterone plus a PDE5I vs PDE5I alone in the short term (6–16 weeks) |
| No one was helped or harmed, with little to no difference in erectile function, sexual quality of life, and treatment withdrawal due to adverse events; very uncertain data on prostate-related events |
Subscribe
From $180- Immediate, unlimited access to all AFP content
- More than 125 CME credits/year
- AAFP app access
- Print delivery available
Issue Access
$59.95- Immediate, unlimited access to this issue's content
- CME credits
- AAFP app access
- Print delivery available
