Am Fam Physician. 2020;102(1):39-48
Author disclosure: No relevant financial affiliations.
The practice of colposcopy, a diagnostic procedure to evaluate for vaginal, vulvar, and cervical dysplasia, has evolved to incorporate patient risk factors for high-grade cervical intraepithelial neoplasia (CIN) and cancer. Changes in cervical cancer screening and guidelines, human papillomavirus (HPV) vaccination recommendations, and colposcopy standards from the American Society for Colposcopy and Cervical Pathology (ASCCP) have implications for all primary care clinicians, not only those who perform colposcopies. Primary care clinicians should offer HPV vaccination to all patients between the ages of nine and 26, in addition to cervical cancer screening and follow-up guidance. Primary care clinicians should recognize the degrees of risk of high-grade CIN and cancer conferred by cytology, HPV subtype, and persistence of HPV infection. Clinicians should address modifiable risk factors such as tobacco use, and provide counseling to patients about colposcopy based on their individual risks. Clinicians should conduct shared decision-making about immediate loop electrosurgical excision procedure vs. colposcopy with multiple biopsies and endocervical sampling for patients with the highest risk of cervical cancer, and for patients who are older than 25 years with at least two of the following: HPV-16, HPV-18, and high-grade squamous intraepithelial lesion cytology. Primary care clinicians should be familiar with the 2019 ASCCP guidelines and develop clinic-based systems to ensure appropriate follow-up of abnormal cytology, positive high-risk HPV testing, diagnosed CIN, and cervical cancer. Patients with an abnormal cervical cancer screening history require surveillance, which differs from routine screening for patients with normal prior screening results. Long-term surveillance is recommended for patients with CIN 2 or worse.
Three recent developments show promise to reduce cervical cancer incidence in the United States. In 2016, the U.S. Food and Drug Administration approved a two-dose series of the 9-valent human papillomavirus (HPV) vaccine for children aged nine to 14.1 For patients aged 15 to 26, a three-dose series is recommended.2 Educating families about two-dose HPV vaccination should lead to improved vaccine initiation rates and the shorter series should improve vaccine completion rates. The U.S. Preventive Services Task Force endorsed HPV-only cervical cancer screening every five years for women 30 and older as an alternative to screening with cytology every three years or cotesting with cytology and HPV every five years.3 HPV self-sampling accuracy is similar to traditional office-based clinician sampling, and it has the potential to improve access to cervical cancer screening.4 Lastly, in 2018, the U.S. Food and Drug Administration expanded its approval of the three-dose 9-valent HPV vaccine to people between the ages of 27 and 45. The Centers for Disease Control and Prevention's Advisory Committee on Immunization Practices recommends shared clinical decision-making for patients in this age group who are not vaccinated or who are undervaccinated who might benefit from HPV vaccination.5 Because older patients are less likely to clear high-risk HPV infections,5 this could decrease cervical cancer incidence.
WHAT IS NEW ON THIS TOPIC
Colposcopy
Recommendations from the American Society for Colposcopy and Cervical Pathology 2019 guidelines for the management of abnormal cervical cancer screening tests and cancer precursors are based on risk, not results.
The American Society for Colposcopy and Cervical Pathology 2017 consensus recommendations for colposcopy practice incorporate a patient's risk factors for high-grade CIN 2 or worse into decision-making about tissue sampling.
Long-term follow-up in a Swedish study of women older than 30 years uncovered no patients with CIN 2 or worse who cleared their high-risk HPV infections and a 100% progression to CIN 2 or worse over 13 years when high-risk HPV persisted.
A high-quality study of 47,000 women undergoing colposcopy found that a random biopsy in the setting of a normal colposcopic impression diagnosed 21% of the total CIN 2 and 19% of CIN 3 or worse, primarily in patients with HPV-16 and HPV-18.
CIN = cervical intraepithelial neoplasia; HPV = human papillomavirus.
HPV vaccination and screening have tremendous potential to save lives; however, it is important to note that 15% to 20% of cervical cancers in the United States are adenocarcinomas, and the incidence is rising.6,7 The association between HPV and adenocarcinoma is less pronounced than for squamous cell carcinoma of the cervix, which accounts for more than 70% of cervical cancers in the United States. Up to one-fourth of adenocarcinomas of the cervix occur in patients who do not have HPV infections.8
Risk-Based Colposcopy
Colposcopy is a diagnostic test used to evaluate vaginal, vulvar, and cervical dysplasia.9 This article is limited to cervical colposcopy. Colposcopy is indicated when the immediate risk of cervical intraepithelial neoplasia (CIN) 2 or worse is 4% or greater, as determined by prior screening results or histology and current high-risk HPV and cytology results. Abnormal-appearing vaginal or cervical tissue should also be evaluated with colposcopy. Although colposcopy has been used in the United States since the 1960s, it was previously informed by cervical cytology, colposcopic impression, and prior histology. In the past 10 to 15 years, testing for high-risk HPV, the etiologic agent in most cases of cervical dysplasia, and high-risk HPV subtypes has been increasingly available.
In 2012 the American Society for Colposcopy and Cervical Pathology (ASCCP) published guidelines for managing abnormal cervical cancer screening tests and cancer precursors that introduced a new concept: using patient risk for progression to cancer and chance of HPV clearance based on age and HPV subtype (HPV-16, HPV-18, and other high-risk HPV strains) to guide clinical decision-making when referring for colposcopy and planning follow-up.10 These recommendations were summarized in algorithms that began with cytology results. In April of 2020, the ASCCP released the 2019 guidelines for managing abnormal cervical cancer screening tests and cancer precursors, which call for a complete shift to risk-based decision-making based on increasing evidence that persistent HPV infection is a primary driver of cervical cancer risk.11 Colposcopy is now recommended for “any combination of history and current test results yielding a 4% or greater probability of finding CIN 3 or worse.”11 The guidelines are accompanied by tables for estimating risk that were developed from a prospective longitudinal cohort of more than 1.5 million patients for more than 10 years in the Kaiser Permanente Northern California health system.
Risk factors for high-grade CIN (Table 112–16) should also inform colposcopy itself, guiding decisions about tissue sampling. In 2017, the ASCCP published recommendations for colposcopy practices that delineate a risk-based approach to the procedure.17 The ASCCP's 2019 guidelines refer back to these recommendations for colposcopy.
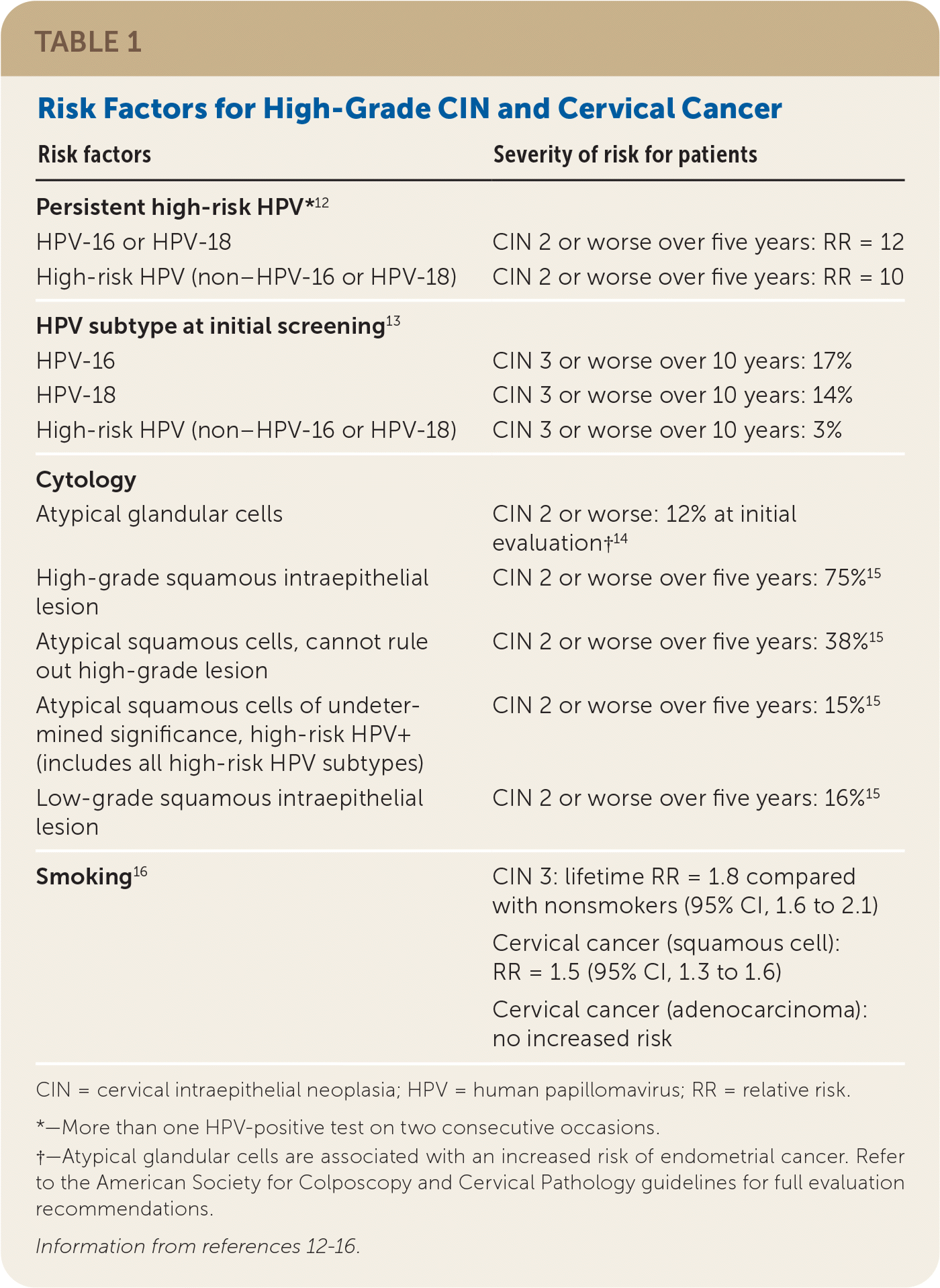
| Risk factors | Severity of risk for patients |
|---|---|
| Persistent high-risk HPV*12 | |
| HPV-16 or HPV-18 | CIN 2 or worse over five years: RR = 12 |
| High-risk HPV (non–HPV-16 or HPV-18) | CIN 2 or worse over five years: RR = 10 |
| HPV subtype at initial screening13 | |
| HPV-16 | CIN 3 or worse over 10 years: 17% |
| HPV-18 | CIN 3 or worse over 10 years: 14% |
| High-risk HPV (non–HPV-16 or HPV-18) | CIN 3 or worse over 10 years: 3% |
| Cytology | |
| Atypical glandular cells | CIN 2 or worse: 12% at initial evaluation†14 |
| High-grade squamous intraepithelial lesion | CIN 2 or worse over five years: 75%15 |
| Atypical squamous cells, cannot rule out high-grade lesion | CIN 2 or worse over five years: 38%15 |
| Atypical squamous cells of undetermined significance, high-risk HPV+ (includes all high-risk HPV subtypes) | CIN 2 or worse over five years: 15%15 |
| Low-grade squamous intraepithelial lesion | CIN 2 or worse over five years: 16%15 |
| Smoking16 | CIN 3: lifetime RR = 1.8 compared with nonsmokers (95% CI, 1.6 to 2.1) |
| Cervical cancer (squamous cell): RR = 1.5 (95% CI, 1.3 to 1.6) | |
| Cervical cancer (adenocarcinoma): no increased risk |
Evidence supporting the ASCCP's new guidelines is plentiful. A 2018 systematic review and meta-analysis of the risk of CIN 2 or worse showed that patients with cytology of low-grade squamous intraepithelial lesion or less who were HPV-16 and HPV-18 negative and had a normal colposcopic impression were at low risk of CIN 2 or worse, whereas patients having two or more of the following—cytology high-grade squamous intraepithelial lesion (HSIL) or worse, HPV-16 or HPV-18, and high-grade colposcopic impression—were at the highest risk of CIN 2 or worse.18 For patients older than 25 years with two or more of the following—HPV-16, HPV-18, and HSIL—the risk of high-grade CIN is so great that patients should be offered the option of immediate loop electrosurgical excision procedure (LEEP) for simultaneous diagnosis and treatment, vs. colposcopy with multiple targeted biopsies.19–21
A persistent HPV infection, defined as more than one HPV-positive test on two separate consecutive occasions, is a predictor of progression to cancer.5 Few long-term studies of high-risk HPV persistence have been conducted, but one Swedish study involving women older than 30 years with HPV at study initiation found no cases of CIN 2 or worse in patients who cleared their HPV infection and 100% progression to CIN 2 or worse (40 out of 40 patients) over 13 years when high-risk HPV persisted.22 In the Kaiser Permanente Northern California health system database of 22,625 patients with a history of HPV-positive/normal cytology results followed by subsequent HPV-negative/normal cytology results, the immediate risk of CIN 3 or worse was 0.01%. Conversely, of 11,990 patients with initial HPV-positive/normal cytology followed by subsequent HPV-positive/normal cytology results, 4.1% had CIN 3 or worse on biopsy.23 In the United States, 56% of cervical cancer diagnoses are made in inadequately screened patients. Another 13% of patients with cervical cancer diagnoses have had errors of follow-up,24 suggesting unchecked gradual disease progression. Clearance of HPV leads to the regression of CIN, with 60% of CIN 2 resolving in patients younger than 30 who clear the virus.25 A 2019 systematic review found that persistence of the same genotype of HPV after treatment for high-grade CIN has a positive predictive value of 44% for posttreatment high-grade CIN.26 Risk factors for persistence include increasing age,5 smoking,27 and immunocompromise.28
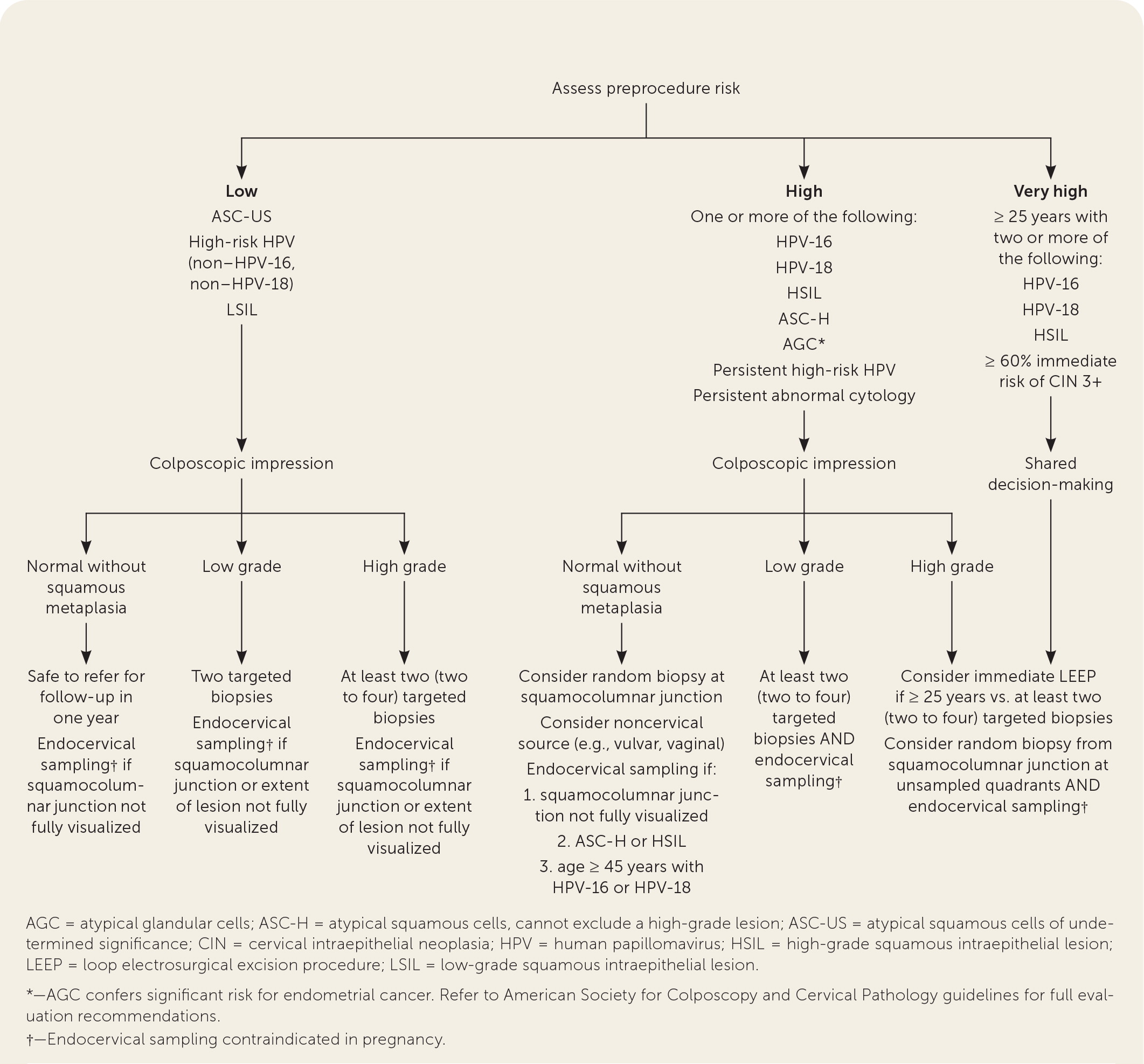
Figure 1 summarizes recommendations for biopsy and endocervical sampling based on the patient's risk of CIN 2 or worse before the procedure combined with the intraprocedural risk determined by the appearance of the cervix during the colposcopy (i.e., colposcopic impression).10,17–21,29–33 This figure may be used by clinicians who perform cervical cancer screening as a guide for counseling patients with abnormal Papanicolaou test results or high-risk HPV results before referral, and by clinicians performing a colposcopy to inform decision-making about tissue sampling.
CERVICAL BIOPSY AND ENDOCERVICAL SAMPLING
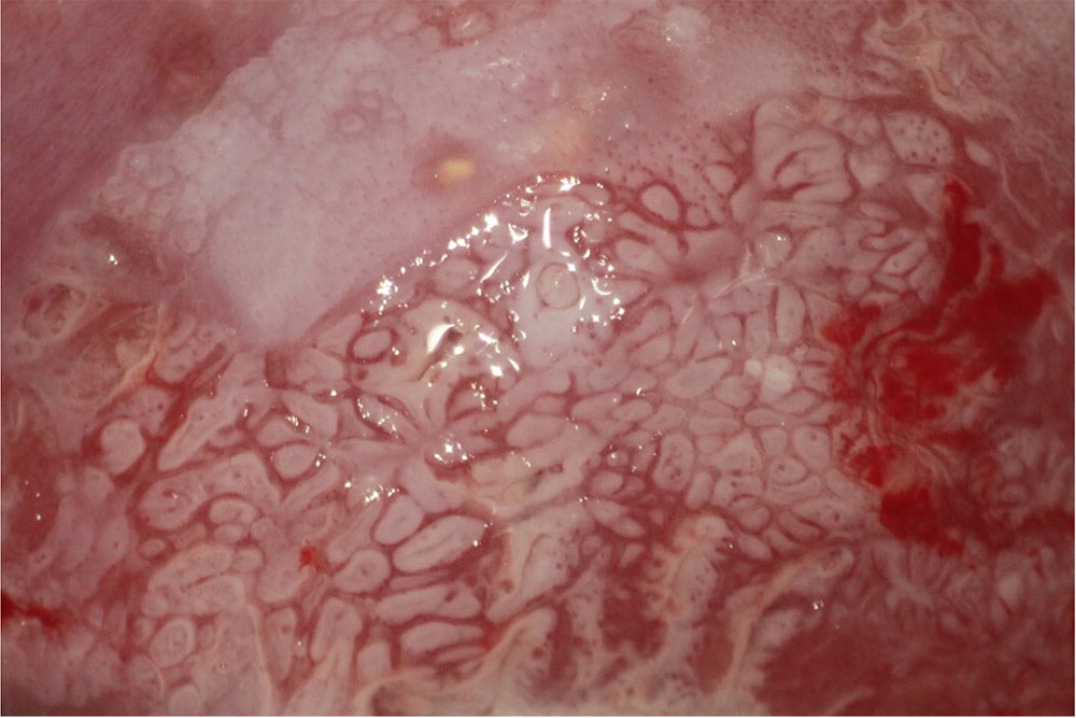
Biopsies should target any lesion present on the cervix.19 Two to four targeted biopsies (i.e., biopsies of abnormal-appearing or acetowhite tissue) within the squamocolumnar junction improve detection of CIN 2 or worse.19,21,29–31 Targeted biopsies are eight to 12 times more likely to uncover CIN 3 or worse than random biopsies.34 For examples of abnormal cervical findings see Figure 2, Figure 3, Figure 4, and Figure 5.
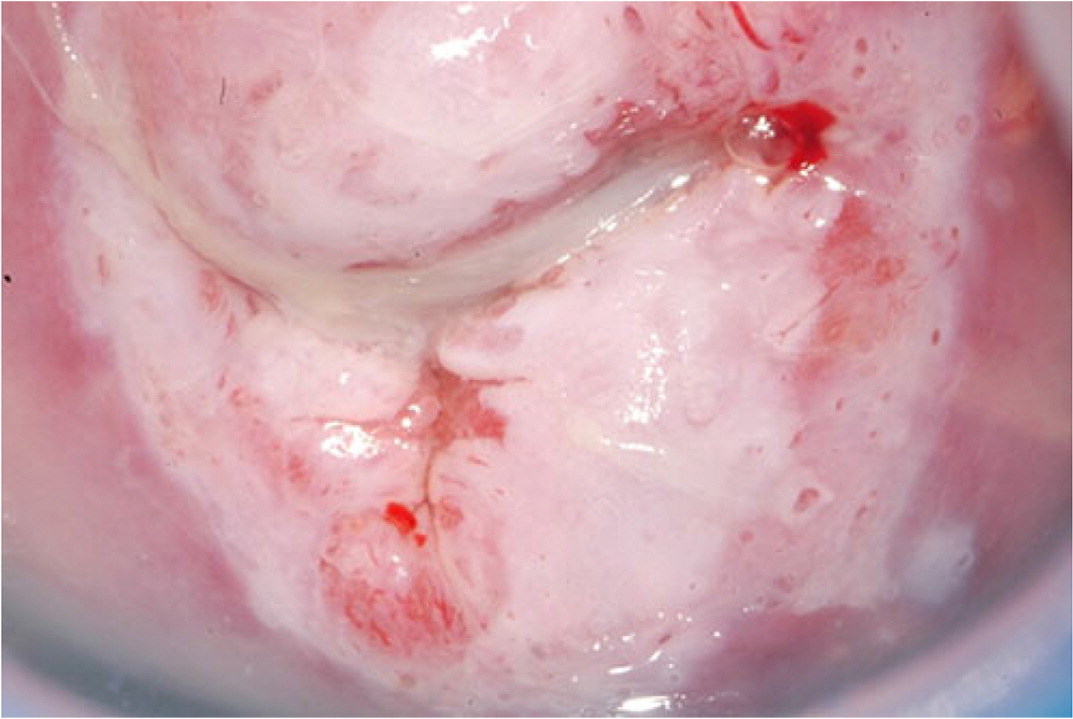
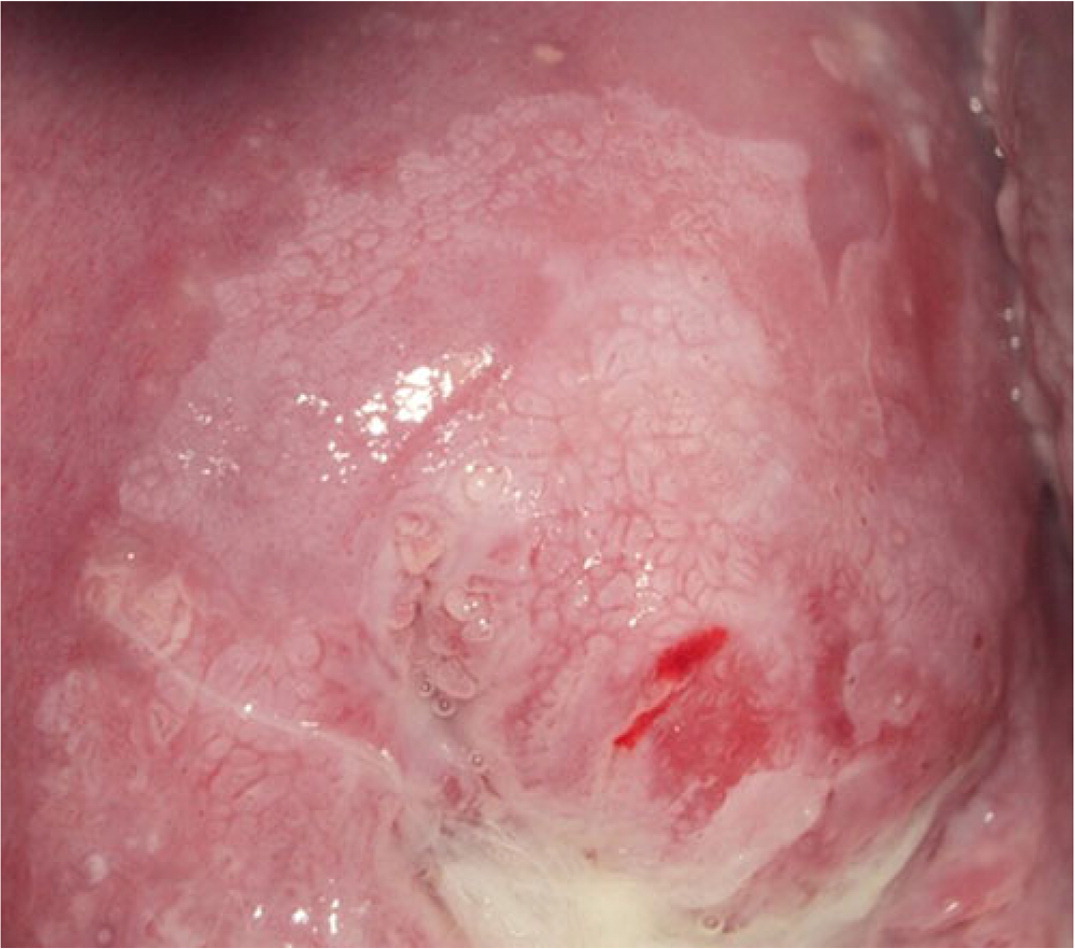
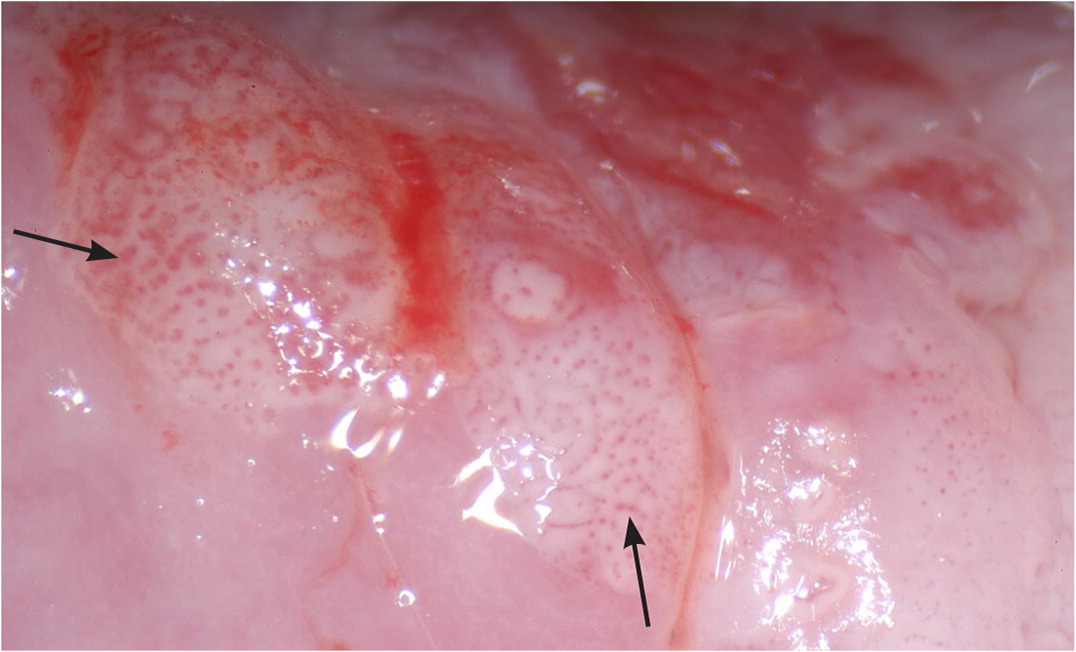
Expert opinion varies about whether random biopsies should be collected and, if so, how many should be collected. One high-quality study of 47,000 women undergoing colposcopy found that a random biopsy in the setting of a normal colposcopic impression diagnosed 21% of the total CIN 2 and 19% of CIN 3 or worse, primarily in patients with HPV-16 and HPV-18.35 Two studies evaluating cervical biopsy found that adding a random biopsy of normal-appearing squamocolumnar junction to targeted biopsy when the colposcopic impression was abnormal increased detection of CIN 2 or worse between 2% and 4.5%.21,31 If there are no lesions and no visible squamous metaplasia during the colposcopy, a random biopsy or biopsies should be considered at the squamocolumnar junction for patients at the highest risk of CIN 2 or worse (Figure 1).10,17–21,29–33 When the colposcopic impression is abnormal, random biopsy at the squamocolumnar junction from unsampled quadrants could be considered, in addition to the recommended two to four targeted biopsies. The ASCCP recommends against performing random biopsies for low-risk patients with normal colposcopic impression and no squamous metaplasia (squamous metaplasia is a normal finding but can be confused with acetowhite changes).17
The 2012 ASCCP guidelines for managing abnormal cervical cancer screening results recommend endocervical sampling for patients with high-grade cytology: HSIL and atypical squamous cells–unable to exclude high grade and whenever the entire squamocolumnar junction cannot be visualized.10 Endocervical sampling should also be performed in patients with two or more of the following: age 45 years or older, HPV-16, and HPV-18, and in any patient who is at high risk with high-grade colposcopic impressions.33 Pregnant patients are excluded from the recommendations above; endocervical sampling is contraindicated in pregnancy.
Endocervical sampling may be performed with a cytobrush or an endocervical curette. The cytobrush technique requires 12 swipes of the endocervical canal while rotating the brush. The endocervical curette should scrape circumferentially from the lower uterine segment to the external os. Sensitivity is similar between the two methods; however, the cytobrush is better tolerated by patients. Curettage should be used in patients who are at high risk or if there is a possibility of invasive disease to obtain stromal information.36,37
Cervical biopsies can be sent together in the same container to minimize cost, because treatment depends on the highest grade lesion detected. The endocervical sample should be sent in a separate container labeled “endocervical sample.” Table 2 highlights the equipment and instruments needed to perform colposcopy.

| 3% to 5% acetic acid, saline, and Lugol solution |
| Array of vaginal specula |
| Medium or large Graves speculum will work for most; adjust the duckbill blades and the overall anteroposterior diameter of the speculum |
| Cotton swabs |
| Endocervical brush |
| Endocervical curette |
| Endocervical speculum to visualize entire squamocolumnar junction |
| Ferric subsulfate (Monsel's solution) or silver nitrate sticks |
| Formalin solution |
| Tenaculum to pull cervix into view or to stabilize cervix for an adequate biopsy |
| Toothpicks to push the ectocervical biopsy sample into a formalin container |
| Variety of biopsy forceps |
| 2-mm biopsy is usually adequate for pathology |
| Single-tooth forceps can be used for most samples |
| Multitoothed forceps are useful for the soft cervix (the teeth on the head can hook and hold the tissue for sampling) |
Colposcopic Impression and Documentation
The 2017 ASCCP consensus colposcopy guidelines include standardized terminology and documentation recommendations that have been endorsed by the American College of Obstetricians and Gynecologists.19 Important components include preprocedure risk assessment, minimum documentation standards, and colposcopic impression.
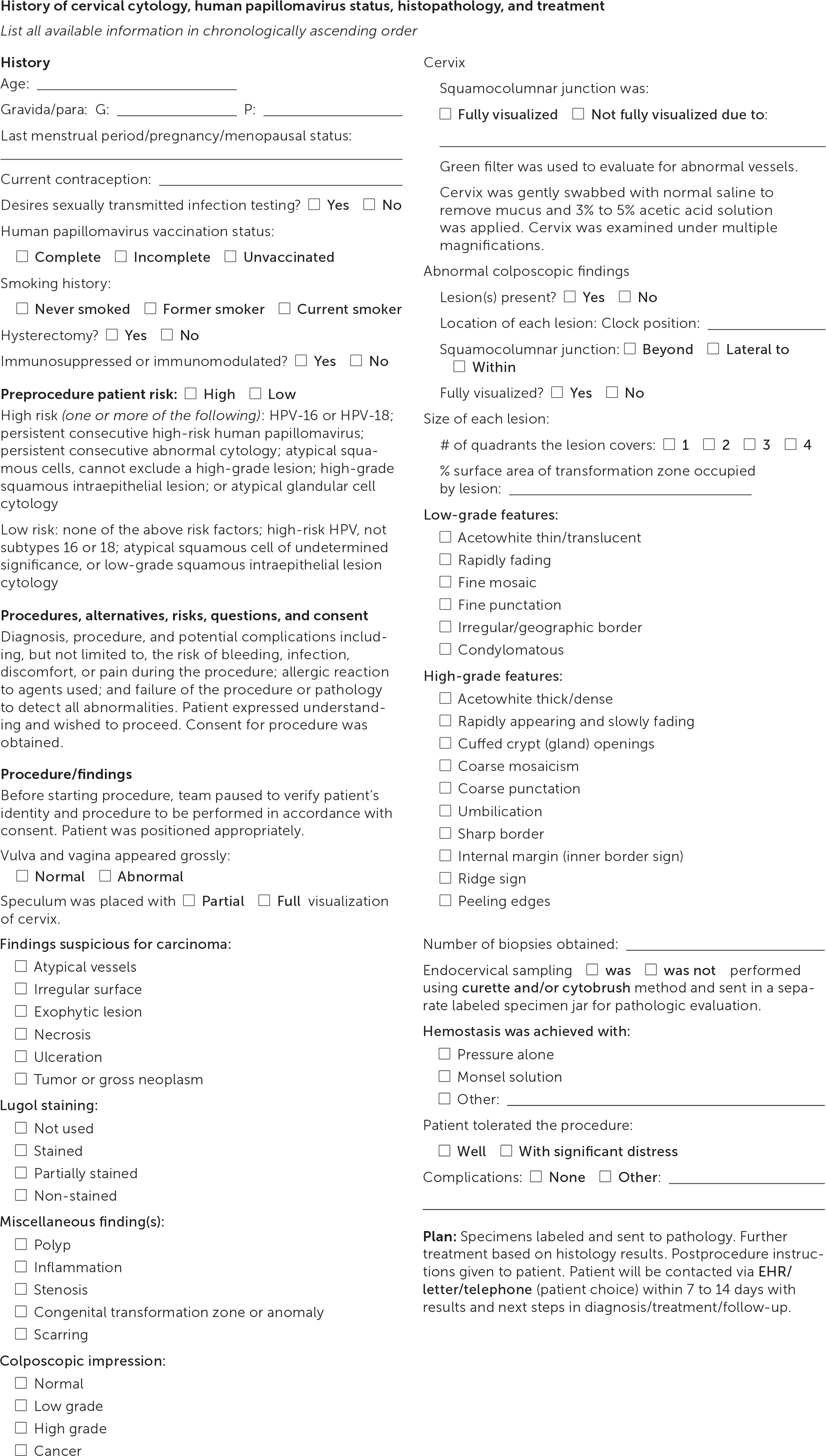
Documentation should note squamocolumnar junction visibility, presence of acetowhitening, presence of lesions, and colposcopic impression.38 An example of the recommended comprehensive documentation, which should include a photo or drawing of the cervix annotated by the clinician,29 is shown in eFigure A. The colposcopic impression is based on the highest-grade feature of any lesion on the cervix.39 Table 3 summarizes lesion characteristics and associated impressions.38 Table 4 offers tips and tricks for colposcopy procedure challenges.
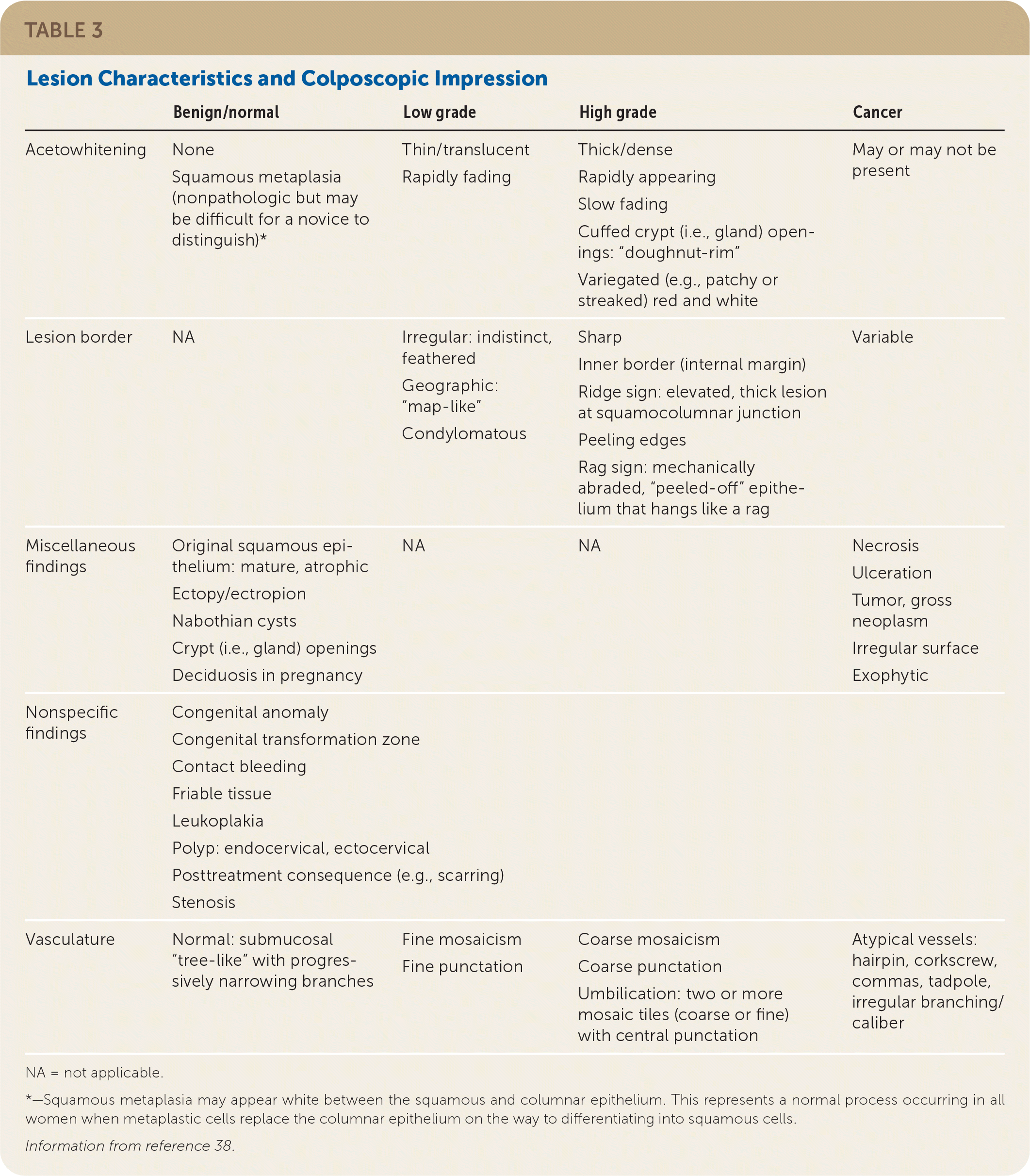
| Benign/normal | Low grade | High grade | Cancer | |
|---|---|---|---|---|
| Acetowhitening | None Squamous metaplasia (nonpathologic but may be difficult for a novice to distinguish)* | Thin/translucent Rapidly fading | Thick/dense Rapidly appearing Slow fading Cuffed crypt (i.e., gland) openings: “doughnut-rim” Variegated (e.g., patchy or streaked) red and white | May or may not be present |
| Lesion border | NA | Irregular: indistinct, feathered Geographic: “map-like” Condylomatous | Sharp Inner border (internal margin) Ridge sign: elevated, thick lesion at squamocolumnar junction Peeling edges Rag sign: mechanically abraded, “peeled-off” epithelium that hangs like a rag | Variable |
| Miscellaneous findings | Original squamous epithelium: mature, atrophic Ectopy/ectropion Nabothian cysts Crypt (i.e., gland) openings Deciduosis in pregnancy | NA | NA | Necrosis Ulceration Tumor, gross neoplasm Irregular surface Exophytic |
| Nonspecific findings | Congenital anomaly Congenital transformation zone Contact bleeding Friable tissue Leukoplakia Polyp: endocervical, ectocervical Posttreatment consequence (e.g., scarring) Stenosis | |||
| Vasculature | Normal: submucosal “tree-like” with progressively narrowing branches | Fine mosaicism Fine punctation | Coarse mosaicism Coarse punctation Umbilication: two or more mosaic tiles (coarse or fine) with central punctation | Atypical vessels: hairpin, corkscrew, commas, tadpole, irregular branching/caliber |
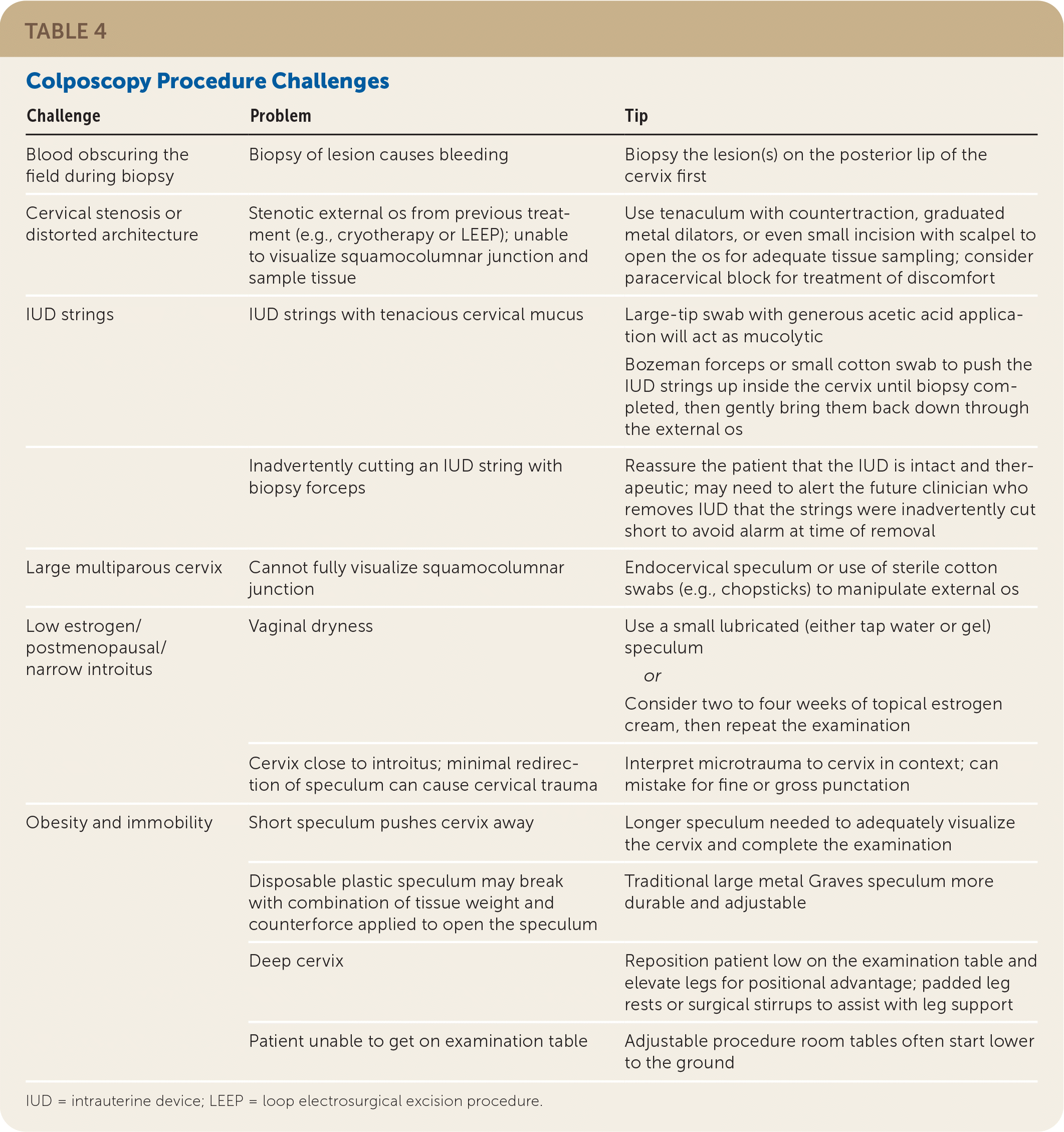
| Challenge | Problem | Tip |
|---|---|---|
| Blood obscuring the field during biopsy | Biopsy of lesion causes bleeding | Biopsy the lesion(s) on the posterior lip of the cervix first |
| Cervical stenosis or distorted architecture | Stenotic external os from previous treatment (e.g., cryotherapy or LEEP); unable to visualize squamocolumnar junction and sample tissue | Use tenaculum with countertraction, graduated metal dilators, or even small incision with scalpel to open the os for adequate tissue sampling; consider paracervical block for treatment of discomfort |
| IUD strings | IUD strings with tenacious cervical mucus | Large-tip swab with generous acetic acid application will act as mucolytic Bozeman forceps or small cotton swab to push the IUD strings up inside the cervix until biopsy completed, then gently bring them back down through the external os |
| Inadvertently cutting an IUD string with biopsy forceps | Reassure the patient that the IUD is intact and therapeutic; may need to alert the future clinician who removes IUD that the strings were inadvertently cut short to avoid alarm at time of removal | |
| Large multiparous cervix | Cannot fully visualize squamocolumnar junction | Endocervical speculum or use of sterile cotton swabs (e.g., chopsticks) to manipulate external os |
| Low estrogen/postmenopausal/narrow introitus | Vaginal dryness | Use a small lubricated (either tap water or gel) speculum or Consider two to four weeks of topical estrogen cream, then repeat the examination |
| Cervix close to introitus; minimal redirection of speculum can cause cervical trauma | Interpret microtrauma to cervix in context; can mistake for fine or gross punctation | |
| Obesity and immobility | Short speculum pushes cervix away | Longer speculum needed to adequately visualize the cervix and complete the examination |
| Disposable plastic speculum may break with combination of tissue weight and counterforce applied to open the speculum | Traditional large metal Graves speculum more durable and adjustable | |
| Deep cervix | Reposition patient low on the examination table and elevate legs for positional advantage; padded leg rests or surgical stirrups to assist with leg support | |
| Patient unable to get on examination table | Adjustable procedure room tables often start lower to the ground |
Guidance for Performing Colposcopy
Colposcopy can be associated with high levels of anxiety that often start after a patient is diagnosed with HPV. Attention to language is important during a colposcopy to ensure the patient's physical and emotional safety and comfort. We recommend the use of trauma-informed language.40 Information about the procedure increases patient knowledge but does not decrease anxiety.41 Visual distractions, in the form of pleasant images on the examination room ceiling or watching a live feed during video colposcopy, have improved patient satisfaction but have not demonstrated consistent improvement of the patient's pain or anxiety during the procedure.42,43
Several randomized controlled trials have evaluated various interventions to decrease the pain associated with cervical biopsies. One trial found no benefits from 800 mg of oral ibuprofen given to the patient before starting the procedure or from topical benzocaine applied two minutes before the biopsy.44 Lidocaine 1% in a dose of 0.5 mL injected at the biopsy site decreases pain during cervical biopsy and endocervical curettage45; however, when lidocaine administration was compared with forced patient cough at the time of biopsy, there was no difference in perception of pain during the procedure.46
Treatment and Follow-up
Treatment options for precancerous lesions of the cervix include cryotherapy, LEEP, and cold knife conization. Excisional treatment is recommended over ablative treatment for CIN 2 and 3.11 The pathology of biopsies and endocervical sampling dictates the treatment and procedures offered. Some primary care clinicians provide the above procedures, and others refer. Adequate tissue samples and detailed descriptions of lesions determine the treatment options. Patients with CIN 2 and 3, cervical cancer, and adenocarcinoma in situ require a minimum of 25 years of ongoing surveillance with HPV testing or cotesting.11
Cervical cancer screening, the detection and treatment of precancerous cervical lesions, and posttreatment follow-up can be performed entirely within a primary care medical home. Modifiable risk factors should always be addressed in primary care (e.g., smoking cessation, HPV vaccination). Clinicians who perform screening should ensure that patients with abnormal test results are managed appropriately and receive follow-up as recommended by the ASCCP guidelines.
This article updates a previous article on this topic by Apgar, et al.47
Data Sources: A PubMed search in Clinical Queries was completed using the key words: colposcopy, cervical cancer screening, human papillomavirus, cervical intraepithelial neoplasia, cervical biopsy, endocervical sampling, and adenocarcinoma of the cervix. We reviewed the evidence summary from Essential Evidence Plus and used multiple sources from that search. We also used the following databases and resources: TRIP database, American Society for Colposcopy and Cervical Pathology (ASCCP), American College of Obstetricians and Gynecologists, the U.S. Preventive Services Task Force, the Seer Database, the Centers for Disease Control and Prevention, and the Cochrane Database of Systematic Reviews. Search dates: September and November 2018, and September 2019. An additional focused review was conducted in April 2020 upon publication of the updated 2019 ASCCP guidelines and associated articles in the Journal of Lower Genital Tract Disease.