
Am Fam Physician. 2022;106(2):202-204
Author disclosure: No relevant financial relationships.
Key Clinical Issue
What are the benefits and harms of cannabis and cannabis-related products for treatment of chronic pain?
Evidence-Based Answer
Some patients with neuropathic pain experience small to moderate short-term pain relief from use of cannabis-related products. This benefit must be weighed against frequent adverse effects.1
An oromucosal spray formulation of cannabis that contains comparable amounts of tetrahydrocannabinol (THC) and cannabidiol (CBD) showed pain-relieving effects. (Strength of Recommendation [SOR]: B, inconsistent or limited-quality patient-oriented evidence.) A moderate pain-relieving effect was found with synthetic oral formulations with a high THC to CBD ratio. (SOR: B, inconsistent or limited-quality patient-oriented evidence.) There was insufficient evidence to assess effects of other treatment formulations (i.e., topical or smoked).1
Adverse effects were more frequent with a higher proportion of THC to CBD. A high THC to CBD ratio in whole plant–based compounds showed the greatest likelihood of patient withdrawal from the study due to adverse effects. (SOR: B, inconsistent or limited-quality patient-oriented evidence.) Dizziness occurred frequently with all formulations.1 (SOR: A, consistent patient-oriented evidence.)
Practice Pointers
Cannabinoid use in the United States has been increasing as more states have legalized cannabis for medical and recreational uses. As of February 2022, there were 37 states, the District of Columbia, Guam, Puerto Rico, and the U.S. Virgin Islands that had approved medical marijuana and cannabis programs.2 Pain relief is the most common reason for patients to seek a prescription for medical cannabis.3
In this Agency for Healthcare Research and Quality (AHRQ) review, neuropathic pain was the most studied type of chronic pain. A total of 20 randomized controlled trials (RCTs; n = 1,776) and seven observational studies (n = 13,095) assessing different cannabinoids were included. Study duration ranged from four to 47 weeks. The main outcomes of interest were pain, overall function, and adverse events. Studies were grouped based on THC to CBD ratio: high THC to CBD, comparable THC to CBD, and low THC to CBD. Compound sources (i.e., plant-based, synthetic, or unspecified) varied among the studies.1
When considering the effects of cannabis on people with chronic pain (defined as symptoms persisting for three to six months or longer), the difference in biological effect of CBD- and THC-based treatments should be a factor.4 CBD lacks the dopaminergic effects of THC and has decreased potential for psychoactive effects.5 The formulations associated with the most benefit generally showed the most adverse effects.
Although effects for an individual patient will vary, the current research does not support widespread use of cannabis for chronic pain. Evidence on whole-plant cannabis, topical CBD, low THC to CBD ratio, other cannabinoids, comparisons with active products, and the impact of cannabis use on the use of opioids in this review was insufficient to draw conclusions. Evidence for other plant-based products such as kratom was also insufficient. Much more research will be needed to determine long-term risks and benefits of using these products for chronic pain. Future use will be determined by identifying formulations that provide sufficient pain relief while minimizing adverse effects.
Despite these limitations, the AHRQ review is consistent with international literature. A 2021 BMJ clinical practice guideline identified weak evidence for the use of noninhaled cannabis for neuropathic, nociceptive, and nociplastic chronic pain relief when standard care was insufficient. Specifically, it noted small improvements in pain and sleep quality accompanied by a minimal increase in physical functioning. The BMJ guideline recommends preparations with comparable amounts of THC and CBD over those with a high ratio of THC to CBD.5
Other studies have acknowledged the potential utility of cannabis products in the treatment of neuropathic pain, particularly forms that have a higher THC content. One review discussed current limitations on the ability to conduct further studies in the United States. This is largely attributed to the Schedule I controlled substance status of THC, resulting in strict U.S. Drug Enforcement Administration restrictions on the amount of cannabis that can be legally grown for research and the locations that are permitted to do so.6
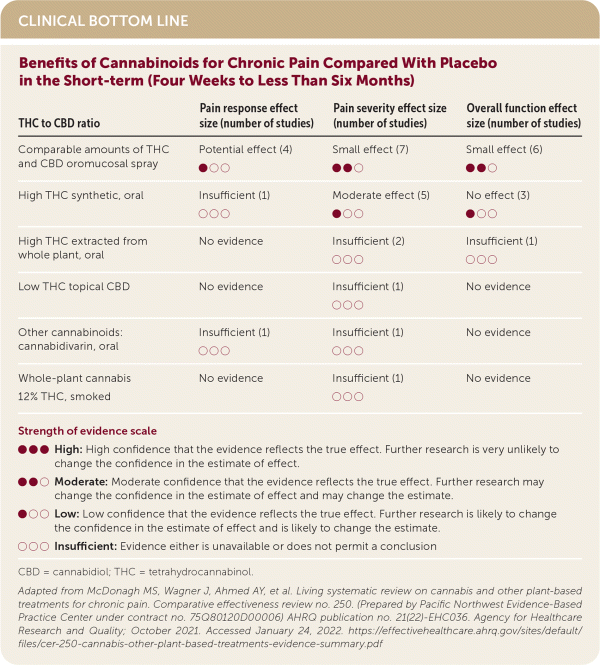
| THC to CBD ratio | Pain response effect size (number of studies) | Pain severity effect size (number of studies) | Overall function effect size (number of studies) |
|---|---|---|---|
| Comparable amounts of THC and CBD oromucosal spray | Potential effect (4) ● ○ ○ | Small effect (7) ● ● ○ | Small effect (6) ● ● ○ |
| High THC synthetic, oral | Insufficient (1) ○ ○ ○ | Moderate effect (5) ● ○ ○ | No effect (3) ● ○ ○ |
| High THC extracted from whole plant, oral | No evidence | Insufficient (2) ○ ○ ○ | Insufficient (1) ○ ○ ○ |
| Low THC topical CBD | No evidence | Insufficient (1) ○ ○ ○ | No evidence |
| Other cannabinoids: cannabidivarin, oral | Insufficient (1) ○ ○ ○ | Insufficient (1) ○ ○ ○ | No evidence |
| Whole-plant cannabis 12% THC, smoked | No evidence | Insufficient (1) ○ ○ ○ | No evidence |
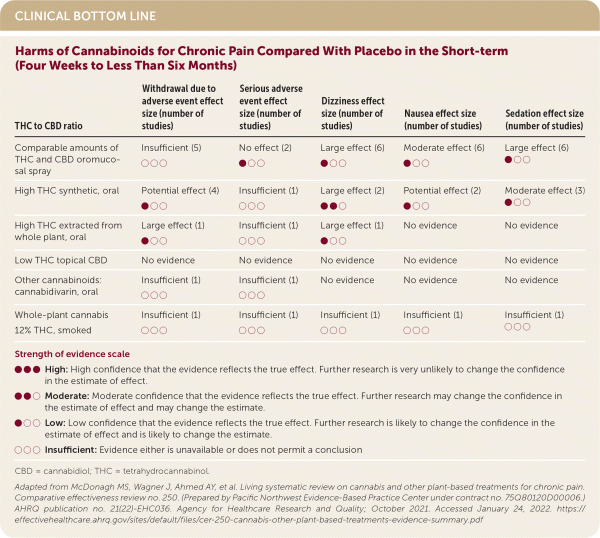
| THC to CBD ratio | Withdrawal due to adverse event effect size (number of studies) | Serious adverse event effect size (number of studies) | Dizziness effect size (number of studies) | Nausea effect size (number of studies) | Sedation effect size (number of studies) |
|---|---|---|---|---|---|
| Comparable amounts of THC and CBD oromucosal spray | Insufficient (5) ○ ○ ○ | No effect (2) ● ○ ○ | Large effect (6) ● ○ ○ | Moderate effect (6) ● ○ ○ | Large effect (6) ● ○ ○ |
| High THC synthetic, oral | Potential effect (4) ● ○ ○ | Insufficient (1) ○ ○ ○ | Large effect (2) ● ● ○ | Potential effect (2) ● ○ ○ | Moderate effect (3) ● ○ ○ |
| High THC extracted from whole plant, oral | Large effect (1) ● ○ ○ | Insufficient (1) ○ ○ ○ | Large effect (1) ● ○ ○ | No evidence | No evidence |
| Low THC topical CBD | No evidence | No evidence | No evidence | No evidence | No evidence |
| Other cannabinoids: cannabidivarin, oral | Insufficient (1) ○ ○ ○ | Insufficient (1) ○ ○ ○ | No evidence | No evidence | No evidence |
| Whole-plant cannabis 12% THC, smoked | Insufficient (1) ○ ○ ○ | Insufficient (1) ○ ○ ○ | Insufficient (1) ○ ○ ○ | Insufficient (1) ○ ○ ○ | Insufficient (1) ○ ○ ○ |
AHRQ’s summary is accompanied by an interpretation by an AFP author that will help guide clinicians in making treatment decisions. For the full review, go to https://effectivehealthcare.ahrq.gov/sites/default/files/related_files/cer-250-cannabis-other-plant-based-treatments-chronic-pain.pdf.
Editor's Note: American Family Physician SOR ratings are different from the AHRQ Strength of Evidence ratings. Dr. Seehusen is an assistant medical editor for AFP.
