This is a corrected version of the article that appeared in print.
Am Fam Physician. 2022;106(3):308-315
Author disclosure: No relevant financial relationships.
Approximately 10 million people worldwide were infected with tuberculosis (TB) in 2019, resulting in 1.4 million deaths. In the United States that same year, there were nearly 9,000 reported cases of TB disease and up to 13 million people were living with latent TB infection (LTBI), which is an asymptomatic, noncommunicable infection caused by Mycobacterium tuberculosis. Without treatment, LTBI will progress to active TB disease in approximately 5% to 10% of affected people. Individuals with symptoms of TB disease warrant testing. The U.S. Preventive Services Task Force recommends testing individuals at increased risk of LTBI with an interferon-gamma release assay or tuberculin skin testing. Because the incidence of LTBI in health care professionals is similar to that of the general population, periodic retesting is not recommended. After a positive test result, chest radiography should be performed and, in patients with suspected pulmonary TB disease, sputum collected for diagnosis. Both suspected and confirmed cases of LTBI and TB disease must be reported to local or state health departments. Preferred treatment regimens for LTBI include isoniazid in combination with rifapentine or rifampin, or rifampin alone for a duration of three and four months, respectively. Treatment of drug-susceptible TB disease includes an eight-week intensive phase with four drugs (isoniazid, rifampin, pyrazinamide, and ethambutol), followed by a continuation phase lasting 18 weeks or more, with two drugs based on susceptibility testing results. Consultation with a TB expert is necessary if there is suspicion or confirmation of drug-resistant TB.
In 2019, approximately 10 million people worldwide were diagnosed with tuberculosis (TB), an infection caused by Mycobacterium tuberculosis, resulting in 1.4 million deaths.1 In the United States, there were nearly 9,000 reported cases of TB disease in 2019, with up to 13 million people living with latent TB infection (LTBI).2 Over the past decade, TB incidence in the United States has decreased by 2% to 3% annually, except in 2020 when the incidence was 20% lower compared with 2019.2,3 The COVID-19 pandemic may have affected the reporting of TB incidence in several ways, including underdiagnosis and a true reduction in the incidence of TB.2
| Clinical recommendation | Evidence rating | Comments |
|---|---|---|
| Screening for LTBI is recommended in individuals at increased risk.5 | B | U.S. Preventive Services Task Force recommendation |
| Interferon-gamma release assay and tuberculin skin testing are the preferred methods of testing for TB in at-risk individuals.6 | C | CDC recommendation |
| Preferred treatment regimens for LTBI are three to four months in duration.18 | C | National Tuberculosis Controllers Association and CDC recommendation |
| In active, drug-susceptible TB disease, four drugs (i.e., isoniazid, rifampin, pyrazinamide, and ethambutol) should be used for an intensive phase of eight weeks. This is followed by a continuation phase of four months with two drugs based on susceptibility test results (i.e., isoniazid and rifampin).24 | C | American Thoracic Society, CDC, and Infectious Diseases Society of America recommendation |
TB is caused by inhalation of respiratory droplets containing M. tuberculosis from a person with active respiratory disease. The M. tuberculosis bacilli multiply in the alveoli and can enter the bloodstream, spreading throughout the body (e.g., brain, larynx, lymph nodes, spine, bone, kidneys). The immune response to TB infection is the formation of granulomas resulting in LTBI. If the immune system cannot control the infection, the bacilli will multiply and progress to TB disease. Without treatment, LTBI will progress to active TB disease in 5% to 10% of affected people.4,5 Risk factors for progression include immunosuppression, diabetes mellitus, intravenous drug use, low body weight, and age younger than five years.6
Which Clinical Situations Warrant TB Testing?

| Employees of, or residents who live or have lived in, congregate settings (e.g., homeless shelters, correctional facilities, nursing homes)* |
| Health care workers who have been exposed to a patient with known TB disease* |
| Infants, children, and adolescents exposed to adults who are known to have latent TB infection or TB disease* |
| Patients known to have HIV, diabetes mellitus, gastrectomy or jejunoileal bypass, low body weight (< 90% of ideal body weight), silicosis, chronic renal failure, leukemia, or cancer of the head, neck, or lungs† |
| Patients taking active immunosuppressive therapy (e.g., tumor necrosis factor–alpha antagonists, systemic corticosteroids [15 mg or more of prednisone per day], immunosuppressive drug therapy following organ transplantation)† |
| People at increased risk of infection because of social determinants, including medically underserved or low-income populations or people who misuse drugs or alcohol*† |
| People born in or who frequently travel to Mexico, the Philippines, Vietnam, India, China, Haiti, Guatemala, sub-Saharan Africa, or other countries with high rates of TB* |
| People who have had contact with someone who has tested positive for or is presumed to have TB disease* |
| People with a history of untreated or inadequately treated TB disease† |
| People with recent Mycobacterium tuberculosis infection (within the past two years)† |
EVIDENCE SUMMARY
Testing should be performed in individuals with symptoms of TB disease and in asymptomatic individuals at increased risk of LTBI and progression to TB disease.5 Symptoms of TB disease include chronic cough (i.e., lasting three weeks or longer), hemoptysis, chest pain, fever, night sweats, anorexia, fatigue, and unexplained weight loss. People at increased risk include those who were born in, or are former residents of, countries with increased TB prevalence (e.g., immigrants, refugees), those who live or have lived in congregate settings (e.g., homeless shelters, correctional facilities), and people with known exposure to TB disease.5–7 People from sub-Saharan Africa and Southeast Asia are particularly at risk of LTBI.7–9 People at low risk of getting TB should not be tested because of the low positive predictive value of testing in low-risk populations.5,6
Initial testing is recommended for all health care professionals upon hire or preplacement. Repeat testing is recommended only for known exposure or based on risk assessments of the health care facility and setting.6 The incidence of LTBI in health care professionals is similar to that of the general population because of a substantial decline in the annual national TB rate in 2017 compared with previous decades.10 A screening questionnaire can be a useful tool in the assessment of health care workers at risk of TB.11
All suspected and confirmed cases of LTBI and TB disease must be reported to local or state health departments. The health department will assist with diagnostic testing, follow-up, and treatment decisions.6
What Are the Recommended TB Tests?
Interferon-gamma release assay (IGRA) and tuberculin skin testing (TST) are recommended options in testing for TB in at-risk individuals.6
EVIDENCE SUMMARY
IGRA testing requires a blood sample. Two IGRA tests are currently approved for use in the United States: QuantiFERON-TB Gold+ (Qiagen) and T-SPOT.TB (Oxford Immunotec). Both indicate immune sensitization to M. tuberculosis.
TST requires an intradermal injection of 0.1 mL of purified protein derivative and is interpreted 48 to 72 hours later. Induration (not erythema) of 15 mm or more is considered positive in patients without risk factors, 10 mm or more is positive for those at low to intermediate risk of progression to TB disease (e.g., past residence in countries where TB disease is common, diabetes, chronic renal failure, alcohol and intravenous drug use, mycobacteriology laboratory workers, age younger than five years, low body weight), and 5 mm or more is positive for patients at increased risk (e.g., immunocompromised, HIV infection, organ transplants, close contact with someone who has a TB infection, clinical or radiographic evidence of current or prior TB infection).6–9,12
Use of TST and IGRA cannot differentiate between LTBI and TB disease or predict the risk of progression from LTBI to TB disease. Chest radiography is indicated when IGRA or TST results are positive.
What Are the Advantages and Disadvantages of TST vs. IGRA?
Advantages of TST include less expense and no need for a laboratory. Disadvantages include the need for more than one visit, trained staff to complete testing, and lower specificity and sensitivity. Advantages of IGRA testing include no requirement for a follow-up visit to interpret results and greater sensitivity and specificity. Disadvantages of IGRA testing include cost, and it is not recommended for use in children younger than two years.32 A comparison of TB tests is discussed in Table 2.12,13,32 [corrected]
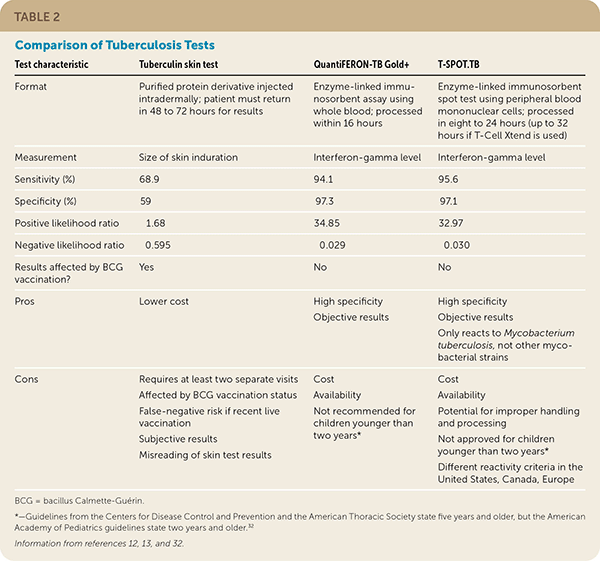
| Test characteristic | Tuberculin skin test | QuantiFERON-TB Gold+ | T-SPOT.TB |
|---|---|---|---|
| Format | Purified protein derivative injected intradermally; patient must return in 48 to 72 hours for results | Enzyme-linked immunosorbent assay using whole blood; processed within 16 hours | Enzyme-linked immunosorbent spot test using peripheral blood mononuclear cells; processed in eight to 24 hours (up to 32 hours if T-Cell Xtend is used) |
| Measurement | Size of skin induration | Interferon-gamma level | Interferon-gamma level |
| Sensitivity (%) | 68.9 | 94.1 | 95.6 |
| Specificity (%) | 59 | 97.3 | 97.1 |
| Positive likelihood ratio | 1.68 | 34.85 | 32.97 |
| Negative likelihood ratio | 0.595 | 0.029 | 0.030 |
| Results affected by BCG vaccination? | Yes | No | No |
| Pros | Lower cost | High specificity Objective results | High specificity Objective results Only reacts to Mycobacterium tuberculosis, not other mycobacterial strains |
| Cons | Requires at least two separate visits Affected by BCG vaccination status False-negative risk if recent live vaccination Subjective results Misreading of skin test results | Cost Availability Not approved for children younger than two years | Cost Availability Potential for improper handling and processing Not approved for children younger than two years Different reactivity criteria in the United States, Canada, Europe |
EVIDENCE SUMMARY
TST is performed without laboratory equipment and is less expensive than IGRA testing, although more personnel time is required. Chronic immunosuppressed states and receiving immunizations with live vaccines within four weeks of TST placement may cause a false-negative result. Reliability of TST is dependent on clinician technique and experience.13
IGRA testing is more convenient for the patient because no return visit is necessary. IGRA test results are objective and not affected by reader variability. IGRA testing is not susceptible to a false-positive result in recipients of the bacillus Calmette-Guérin vaccine, but it does have a false-negative risk similar to that of TST performed in immunosuppressed persons.12,14 For a century, the bacillus Calmette-Guérin vaccine has been used in many parts of the world (i.e., Mexico, South America, Africa, Asia, and Western Europe) in the general population to prevent TB infection.15 It is also used in special populations in North America, Eastern Europe, and Australia.
How Is TB Disease Distinguished From LTBI?
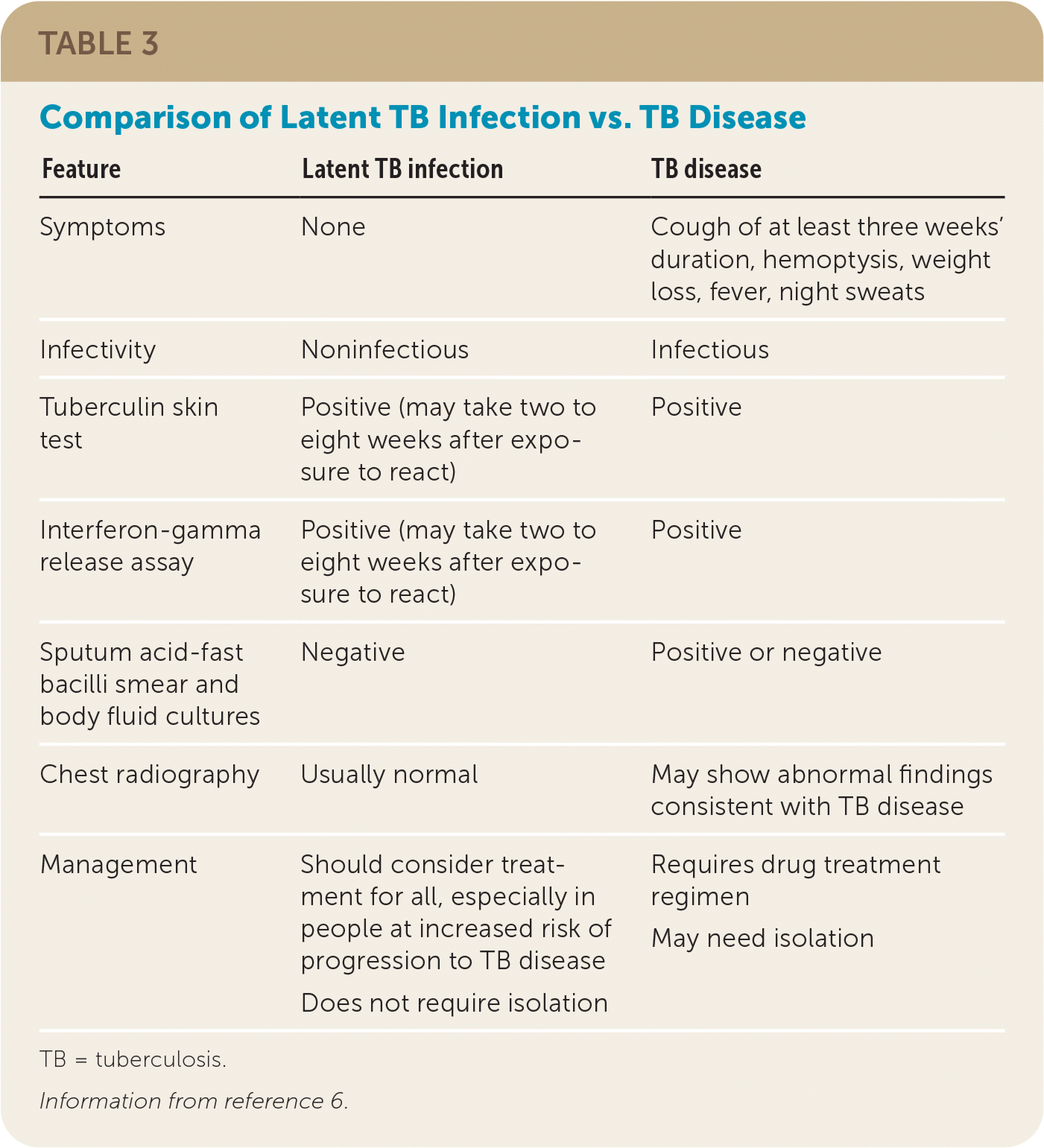
| Feature | Latent TB infection | TB disease |
|---|---|---|
| Symptoms | None | Cough of at least three weeks' duration, hemoptysis, weight loss, fever, night sweats |
| Infectivity | Noninfectious | Infectious |
| Tuberculin skin test | Positive (may take two to eight weeks after exposure to react) | Positive |
| Interferon-gamma release assay | Positive (may take two to eight weeks after exposure to react) | Positive |
| Sputum acid-fast bacilli smear and body fluid cultures | Negative | Positive or negative |
| Chest radiography | Usually normal | May show abnormal findings consistent with TB disease |
| Management | Should consider treatment for all, especially in people at increased risk of progression to TB disease Does not require isolation | Requires drug treatment regimen May need isolation |
EVIDENCE SUMMARY
People with LTBI are asymptomatic, noninfectious, and have chest radiography without findings suggestive of TB disease. Those with TB disease are symptomatic and often contagious. Manifestations of TB disease may include respiratory and constitutional symptoms, abnormal results on chest radiography, and positive sputum smear or culture.
In addition to chest radiography, three sputum specimens for acid-fast bacilli smear microscopy, diagnostic nucleic acid amplification test, and cultures are required in patients with suspected pulmonary TB disease. When extrapulmonary TB is suspected, the specimen should be obtained from the site of infection (e.g., lymph node, urine, pleural fluid, cerebrospinal fluid, bone marrow). Additional testing for drug susceptibility is completed on all positive cultures.12 New methods for whole genome sequencing of M. tuberculosis samples are now widely used in areas of the world with limited resources for laboratory-based culture testing. These tests have similar sensitivity to cultures and provide information about genetic mutations that predict drug resistance.16
What Are the Standard Treatment Recommendations for LTBI?
There are three preferred regimens for the treatment of LTBI: isoniazid plus rifapentine (Priftin), rifampin alone, or isoniazid plus rifampin. Alternative treatment options involve isoniazid monotherapy.17,18 Table 4 summarizes the preferred and alternative treatment options for LTBI.14,17,18 Standard treatment recommendations are modified if a patient has been exposed to someone with drug-resistant TB.
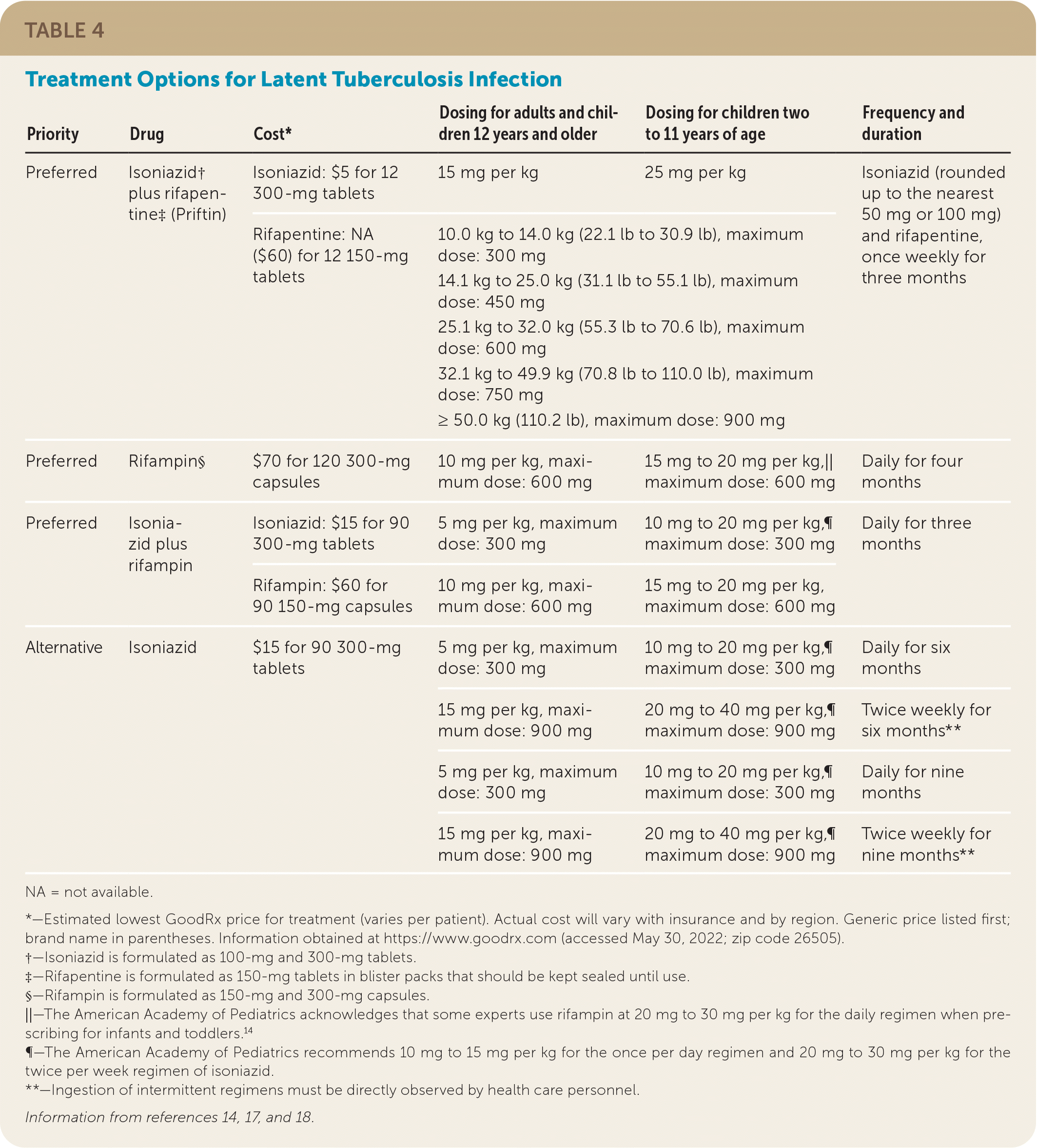
| Priority | Drug | Cost* | Dosing for adults and children 12 years and older | Dosing for children two to 11 years of age | Frequency and duration |
|---|---|---|---|---|---|
| Preferred | Isoniazid† plus rifapentine‡ (Priftin) | Isoniazid: $5 for 12 300-mg tablets | 15 mg per kg | 25 mg per kg | Isoniazid (rounded up to the nearest 50 mg or 100 mg) and rifapentine, once weekly for three months |
| Rifapentine: NA ($60) for 12 150-mg tablets | 10.0 kg to 14.0 kg (22.1 lb to 30.9 lb), maximum dose: 300 mg 14.1 kg to 25.0 kg (31.1 lb to 55.1 lb), maximum dose: 450 mg 25.1 kg to 32.0 kg (55.3 lb to 70.6 lb), maximum dose: 600 mg 32.1 kg to 49.9 kg (70.8 lb to 110.0 lb), maximum dose: 750 mg ≥ 50.0 kg (110.2 lb), maximum dose: 900 mg | ||||
| Preferred | Rifampin§ | $70 for 120 300-mg capsules | 10 mg per kg, maximum dose: 600 mg | 15 mg to 20 mg per kg,|| maximum dose: 600 mg | Daily for four months |
| Preferred | Isoniazid plus rifampin | Isoniazid: $15 for 90 300-mg tablets | 5 mg per kg, maximum dose: 300 mg | 10 mg to 20 mg per kg,¶ maximum dose: 300 mg | Daily for three months |
| Rifampin: $60 for 90 150-mg capsules | 10 mg per kg, maximum dose: 600 mg | 15 mg to 20 mg per kg, maximum dose: 600 mg | |||
| Alternative | Isoniazid | $15 for 90 300-mg tablets | 5 mg per kg, maximum dose: 300 mg | 10 mg to 20 mg per kg,¶ maximum dose: 300 mg | Daily for six months |
| 15 mg per kg, maximum dose: 900 mg | 20 mg to 40 mg per kg,¶ maximum dose: 900 mg | Twice weekly for six months** | |||
| 5 mg per kg, maximum dose: 300 mg | 10 mg to 20 mg per kg,¶ maximum dose: 300 mg | Daily for nine months | |||
| 15 mg per kg, maximum dose: 900 mg | 20 mg to 40 mg per kg,¶ maximum dose: 900 mg | Twice weekly for nine months** | |||
EVIDENCE SUMMARY
All cases of LTBI and TB disease should be reported to local or state health departments, which can provide valuable resources for contact tracing and support to ensure completion of treatment. Guidelines preferentially recommend three- to four-month drug regimens, all of which include a rifamycin-based medication, rather than the previous standard of six or nine months of monotherapy with isoniazid.18 The Centers for Disease Control and Prevention recommends initiating treatment of LTBI after excluding the possibility of TB disease.17 The current treatment guidelines consider the benefits to patients and the public, the complexity of the regimens, potential for toxicity, and costs. Based on these considerations and the quality of evidence, three preferred regimens emerged: (1) 12 weeks of isoniazid plus rifapentine once per week; (2) four months of rifampin once per day; and (3) three months of isoniazid plus rifampin once per day.
Drug-drug interactions, especially with rifampin, may limit the ability to use shorter, preferred options. Alternative regimens use single-drug isoniazid, in a once per day or twice per week dosage for six or nine months. Intermittent regimens must be given under direct observation.
Isoniazid with rifapentine taken once per week for 12 weeks results in better adherence and is as safe and effective in treating LTBI as taking isoniazid once per day for nine months, which has a completion rate of less than 60%.19–22 In patients who have increased risk of progression to TB disease, taking rifampin once per day for four months was not inferior to taking isoniazid monotherapy for nine months because of equivalent effectiveness, improved adherence, and safety.23
What Is the Standard Treatment Regimen for TB Disease?
Treatment of TB disease is typically done in consultation with the local public health department. Standard treatment for drug-susceptible TB disease includes an eight-week intensive phase with isoniazid, rifampin, pyrazinamide, and ethambutol, and is followed by a three- to four-month continuation phase with isoniazid and rifampin (Table 5).24
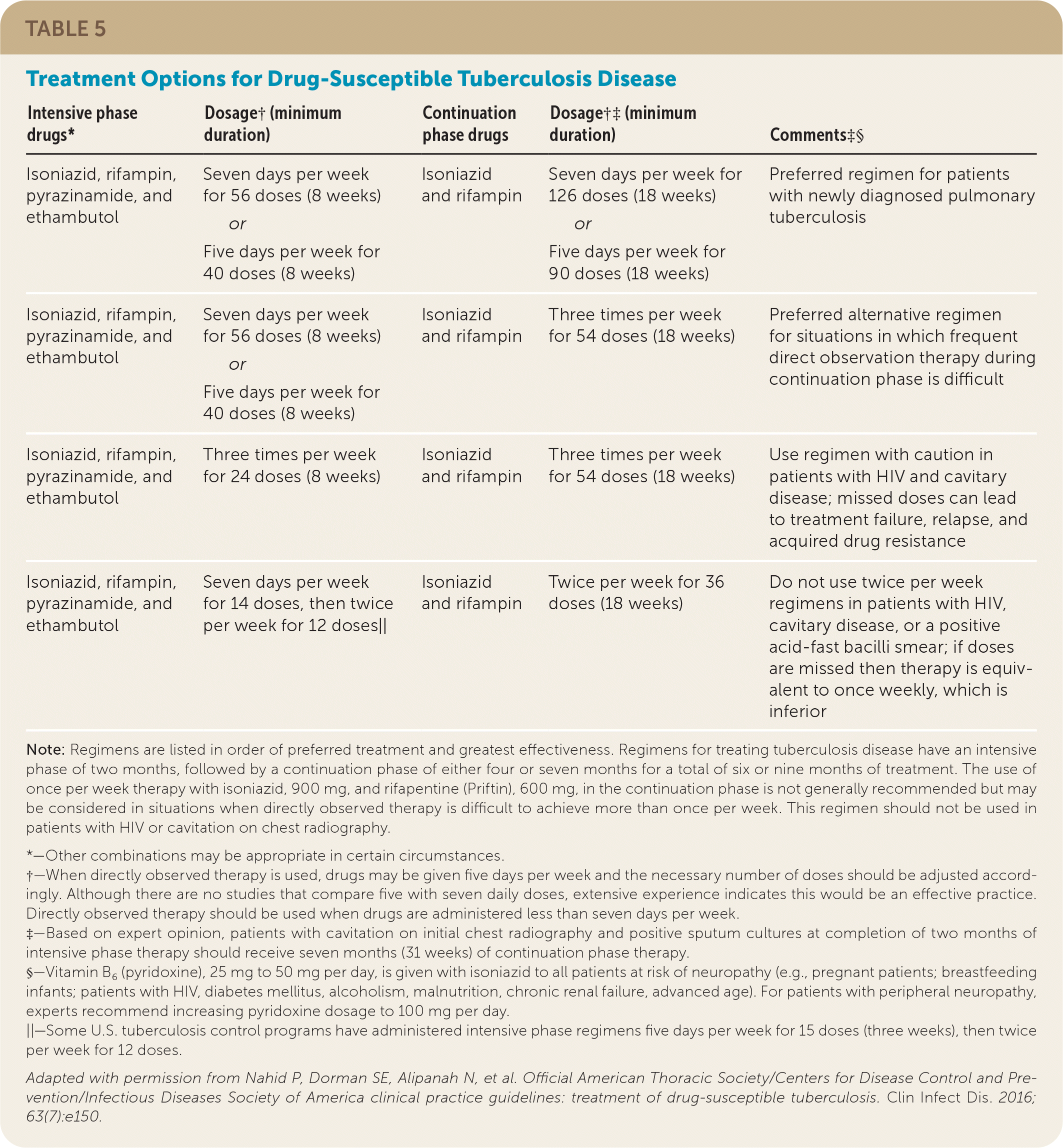
| Intensive phase drugs* | Dosage†(minimum duration) | Continuation phase drugs | Dosage†‡(minimum duration) | Comments‡§ |
|---|---|---|---|---|
| Isoniazid, rifampin, pyrazinamide, and ethambutol | Seven days per week for 56 doses (8 weeks) or Five days per week for 40 doses (8 weeks) | Isoniazid and rifampin | Seven days per week for 126 doses (18 weeks) or Five days per week for 90 doses (18 weeks) | Preferred regimen for patients with newly diagnosed pulmonary tuberculosis |
| Isoniazid, rifampin, pyrazinamide, and ethambutol | Seven days per week for 56 doses (8 weeks) or Five days per week for 40 doses (8 weeks) | Isoniazid and rifampin | Three times per week for 54 doses (18 weeks) | Preferred alternative regimen for situations in which frequent direct observation therapy during continuation phase is difficult |
| Isoniazid, rifampin, pyrazinamide, and ethambutol | Three times per week for 24 doses (8 weeks) | Isoniazid and rifampin | Three times per week for 54 doses (18 weeks) | Use regimen with caution in patients with HIV and cavitary disease; missed doses can lead to treatment failure, relapse, and acquired drug resistance |
| Isoniazid, rifampin, pyrazinamide, and ethambutol | Seven days per week for 14 doses, then twice per week for 12 doses|| | Isoniazid and rifampin | Twice per week for 36 doses (18 weeks) | Do not use twice per week regimens in patients with HIV, cavitary disease, or a positive acid-fast bacilli smear; if doses are missed then therapy is equivalent to once weekly, which is inferior |
EVIDENCE SUMMARY
Empiric treatment of TB disease begins with a regimen of four drugs. The preferred course of therapy for treating adults and children with TB disease known or believed to be drug-susceptible consists of an eight-week intensive phase using isoniazid, rifampin, pyrazinamide, and ethambutol.24 If susceptibility testing confirms sensitivity of the M. tuberculosis isolate to both isoniazid and rifampin, ethambutol can be stopped, completing the intensive phase with isoniazid, rifampin, and pyrazinamide.
The intensive phase is followed by a four-month continuation phase consisting of isoniazid plus rifampin.24 Vitamin B6 (pyridoxine) should be given with isoniazid to people at risk of neuropathy (e.g., pregnant patients; breastfeeding infants; older adults; patients with HIV, diabetes, alcoholism, malnutrition, or chronic renal failure). The preferred frequency of dosing is once per day; however, directly observed therapy five days a week is an acceptable alternative to seven days per week of self-administered therapy.
Baseline and monthly follow-up evaluations are recommended. The results of acid-fast bacilli smears and cultures determine the duration of the continuation phase and, therefore, are critical to appropriate management. Acid-fast bacilli smears and cultures should be obtained monthly until two consecutive specimens are negative. Additional information on monitoring and adverse effects of TB therapy is provided in eTable A.
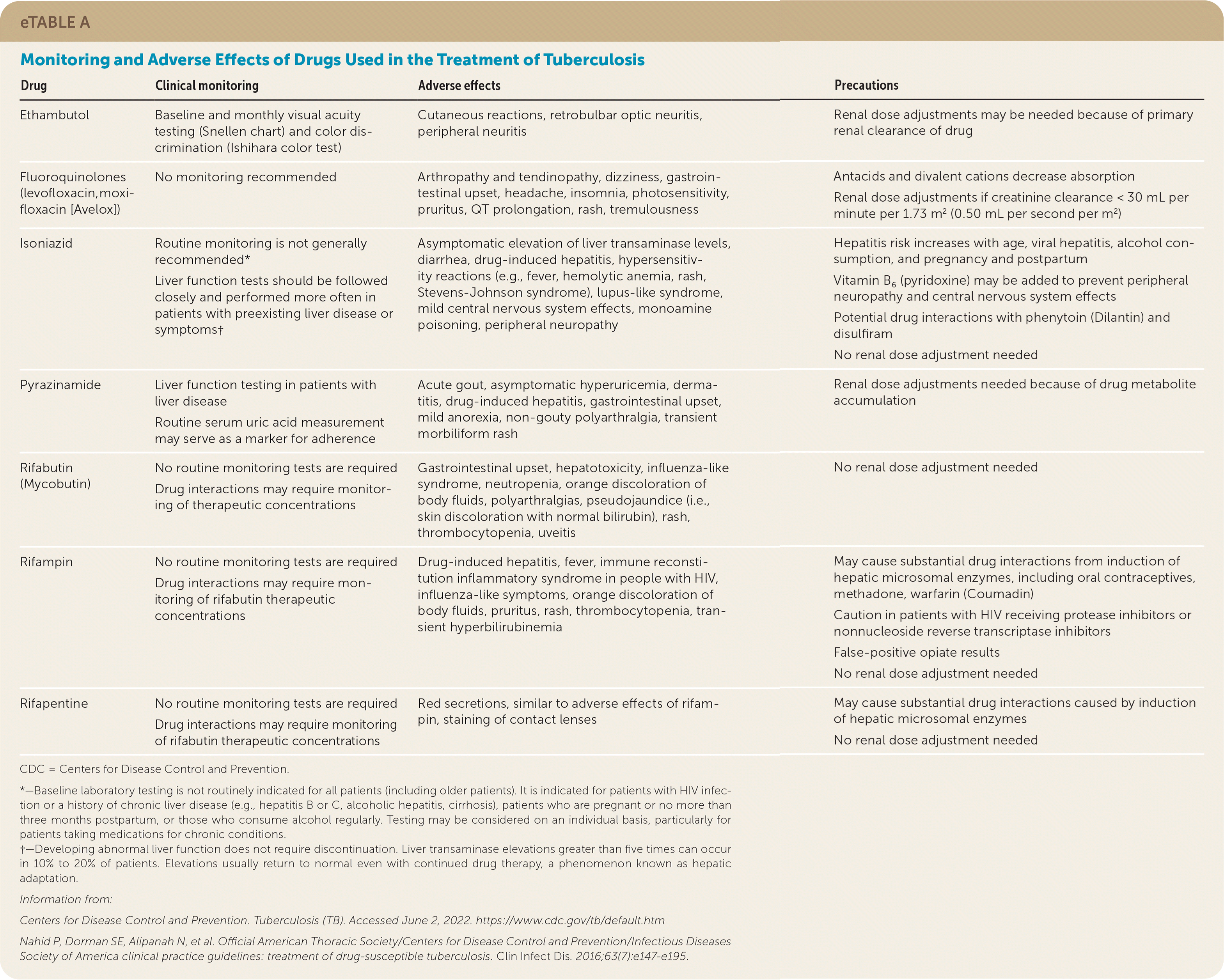
| Drug | Clinical monitoring | Adverse effects | Precautions |
|---|---|---|---|
| Ethambutol | Baseline and monthly visual acuity testing (Snellen chart) and color discrimination (Ishihara color test) | Cutaneous reactions, retrobulbar optic neuritis, peripheral neuritis | Renal dose adjustments may be needed because of primary renal clearance of drug |
| Fluoroquinolones (levofloxacin, moxifloxacin [Avelox]) | No monitoring recommended | Arthropathy and tendinopathy, dizziness, gastrointestinal upset, headache, insomnia, photosensitivity, pruritus, QT prolongation, rash, tremulousness | Antacids and divalent cations decrease absorption Renal dose adjustments if creatinine clearance < 30 mL per minute per 1.73 m2 (0.50 mL per second per m2) |
| Isoniazid | Routine monitoring is not generally recommended* Liver function tests should be followed closely and performed more often in patients with preexisting liver disease or symptoms† | Asymptomatic elevation of liver transaminase levels, diarrhea, drug-induced hepatitis, hypersensitivity reactions (e.g., fever, hemolytic anemia, rash, Stevens-Johnson syndrome), lupus-like syndrome, mild central nervous system effects, monoamine poisoning, peripheral neuropathy | Hepatitis risk increases with age, viral hepatitis, alcohol consumption, and pregnancy and postpartum Vitamin B6 (pyridoxine) may be added to prevent peripheral neuropathy and central nervous system effects Potential drug interactions with phenytoin (Dilantin) and disulfiram No renal dose adjustment needed |
| Pyrazinamide | Liver function testing in patients with liver disease Routine serum uric acid measurement may serve as a marker for adherence | Acute gout, asymptomatic hyperuricemia, dermatitis, drug-induced hepatitis, gastrointestinal upset, mild anorexia, non-gouty polyarthralgia, transient morbiliform rash | Renal dose adjustments needed because of drug metabolite accumulation |
| Rifabutin (Mycobutin) | No routine monitoring tests are required Drug interactions may require monitoring of therapeutic concentrations | Gastrointestinal upset, hepatotoxicity, influenza-like syndrome, neutropenia, orange discoloration of body fluids, polyarthralgias, pseudojaundice (i.e., skin discoloration with normal bilirubin), rash, thrombocytopenia, uveitis | No renal dose adjustment needed |
| Rifampin | No routine monitoring tests are required Drug interactions may require monitoring of rifabutin therapeutic concentrations | Drug-induced hepatitis, fever, immune reconstitution inflammatory syndrome in people with HIV, influenza-like symptoms, orange discoloration of body fluids, pruritus, rash, thrombocytopenia, transient hyperbilirubinemia | May cause substantial drug interactions from induction of hepatic microsomal enzymes, including oral contraceptives, methadone, warfarin (Coumadin) Caution in patients with HIV receiving protease inhibitors or nonnucleoside reverse transcriptase inhibitors False-positive opiate results No renal dose adjustment needed |
| Rifapentine | No routine monitoring tests are required Drug interactions may require monitoring of rifabutin therapeutic concentrations | Red secretions, similar to adverse effects of rifampin, staining of contact lenses | May cause substantial drug interactions caused by induction of hepatic microsomal enzymes No renal dose adjustment needed |
Interruptions in TB therapy can impact treatment, such as extending the duration. Experience with SARS-CoV-2 infection in patients with LTBI and TB disease remains limited, but it is anticipated that they may have poorer treatment outcomes, especially if TB therapy is interrupted. Patients who have TB should be vaccinated for and follow precautions to be protected from COVID-19 while continuing TB therapy as prescribed.25
The treatment of drug-resistant isolates of M. tuberculosis is more complex and includes additional molecular and phenotypic diagnostic tests to determine susceptibilities, the use of second-line drugs, and prolonged treatment durations. The World Health Organization recommendations are rapidly changing the modern treatment of TB disease with the use of fluoroquinolones for isoniazid resistance and newer medications such as bedaquiline (Sirturo) or delamanid (Deltyba; not available in the United States) in cases of rifampin resistance.1,26 A TB expert should be consulted when there is suspicion or confirmation of drug-resistant TB disease.27 Recommendations in special populations (i.e., patients with HIV, extrapulmonary TB, culture-negative pulmonary TB, advanced age, renal or hepatic disease, or who are pregnant or breastfeeding) are beyond the scope of this review.
This article updates previous articles on this topic by Jerant, et al.28; Potter, et al.29; Inge and Wilson30; Hauck, et al.31; and Hartman-Adams, et al.4
Data Sources: The primary resource used to identify literature was PubMed. Search terms included Mycobacterium tuberculosis, latent tuberculosis infection, TB disease, TST, IGRA testing for TB, and treatment of TB. Essential Evidence Plus and article reference lists were reviewed to identify further sources. The search included meta-analyses and reviews. The Centers for Disease Control and Prevention website was also searched using the terms above. Search dates: July 26, 2021; August 6, 2021; August 22, 2021; January 2, 2022; and May 30, 2022.