
Am Fam Physician. 2023;107(2):165-172
Author disclosure: No relevant financial relationships.
Peptic ulcer disease is common, affecting 1 out of 12 people in the United States. Approximately 1 in 5 peptic ulcers is associated with Helicobacter pylori infection, with most of the rest due to nonsteroidal anti-inflammatory drug (NSAID) use. The combination of H. pylori infection and NSAID use synergistically increases the risk of bleeding ulcers more than sixfold. The H. pylori test-and-treat strategy is the mainstay of outpatient management. Patients younger than 60 years who have dyspepsia without alarm symptoms should be tested and, if positive, treated to eradicate the infection. If negative, they should be treated empirically with a proton pump inhibitor (PPI). Esophagogastroduodenoscopy is recommended for patients 60 years and older with new symptoms and for anyone with alarm symptoms. Noninvasive testing for H. pylori using a urea breath test or stool antigen test is preferred. Bismuth quadruple therapy or concomitant therapy (nonbismuth quadruple therapy) is the preferred first-line treatment for eradication because of increasing clarithromycin resistance. To lower the risk of ulcers associated with long-term NSAID use, clinicians should consider coadministering a PPI or substituting an NSAID with less effect on gastric mucosa, such as celecoxib. Eradicating H. pylori in NSAID users reduces the likelihood of peptic ulcers by one-half. Potential risks of long-term PPI use include fractures, interaction with antiplatelet medications, chronic kidney disease, Clostridioides difficile infection, dementia, and magnesium, calcium, and vitamin B12 micronutrient deficiencies.
Upper gastrointestinal symptoms such as dyspepsia (i.e., epigastric discomfort, including pain, fullness, or heartburn) are common in the outpatient setting.1,2 Although dyspepsia often occurs without underlying pathology, it can indicate the presence of peptic ulcer disease (PUD). Patients with PUD have peptic ulcers, typically in the stomach and proximal duodenum, resulting from injury caused by pepsin and gastric acid secretion.3 These ulcers can lead to epigastric pain and may be associated with bloating, abdominal fullness, nausea, or early satiety.4 Approximately 1 out of 12 people in the United States is affected by PUD.4
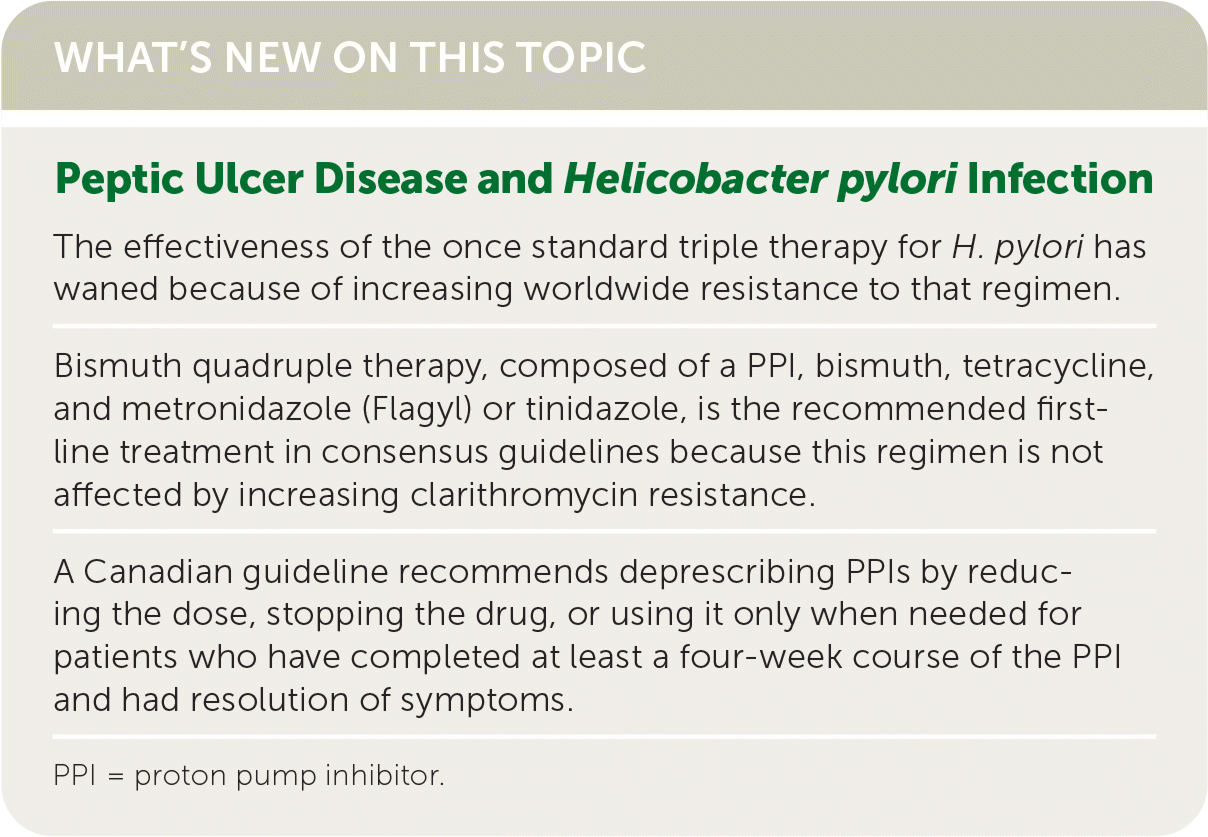
| The effectiveness of the once standard triple therapy for H. pylori has waned because of increasing worldwide resistance to that regimen. |
| Bismuth quadruple therapy, composed of a PPI, bismuth, tetracycline, and metronidazole (Flagyl) or tinidazole, is the recommended first-line treatment in consensus guidelines because this regimen is not affected by increasing clarithromycin resistance. |
| A Canadian guideline recommends deprescribing PPIs by reducing the dose, stopping the drug, or using it only when needed for patients who have completed at least a four-week course of the PPI and had resolution of symptoms. |

| Recommendation | Sponsoring organization |
|---|---|
| Do not request serology for Helicobacter pylori. Use the stool antigen test or breath test instead. | American Society for Clinical Pathology |
What are Risk Factors for PUD?
EVIDENCE SUMMARY
Historically, most peptic ulcers have been associated with H. pylori infection, but there is some evidence that this is changing.6 A cross-sectional study of more than 1 million patients undergoing esophagogastroduodenoscopy, or upper endoscopy, from 2009 to 2018 found that only one-fourth of duodenal ulcers and one-sixth of gastric ulcers were associated with H. pylori infection.5
NSAID use increases the risk of PUD, with a moderate increase for those taking a single drug in this class (odds ratio [OR] = 1.64; 95% CI, 1.43 to 1.87) and a dramatic increase for those taking multiple drugs (OR = 3.82; 95% CI, 2.16 to 6.73).7 Risk increases with duration of use. The OR is 2.46 (95% CI, 1.92 to 3.16) when taken for three years or longer vs. 1.07 (95% CI, 0.84 to 1.35) when taken for less than three months.7 The increased risk of PUD with aspirin use is similar to that seen with other NSAIDs (OR = 1.54; 95% CI, 1.37 to 1.74), whereas the risk is greater with dual antiplatelet therapy using aspirin and clopidogrel (OR = 2.62; 95% CI, 1.12 to 6.11) regardless of duration or aspirin dose.7
A meta-analysis demonstrated that concurrent H. pylori infection and NSAID use synergistically increased the risk of peptic ulcers and bleeding peptic ulcers.8 People with H. pylori infection who used NSAIDs had a 60-fold greater risk of developing peptic ulcers than noninfected people who did not use NSAIDs, and the combination increased the risk of ulcer bleeding more than sixfold.8
How Is PUD Diagnosed?
The main symptom of PUD is a gnawing or burning, episodic, nonradiating epigastric pain that is worsened by eating in those with gastric ulcers and worsened by an empty stomach (two to three hours after meals or at night) and relieved by food or antacids in those with duodenal ulcers.9,10 PUD may also be associated with bloating, abdominal fullness, nausea, or early satiety.4 History and physical examination are important to identify those who may have PUD, including a bleeding ulcer. However, a systematic review found that neither clinical impression nor computer models could adequately distinguish organic from functional disease because of their similar presentations.11 The most accurate test for diagnosing PUD is upper endoscopy, but this test is invasive and costly.
EVIDENCE SUMMARY
The clinician's clinical impression for PUD is modestly helpful in identifying which patients with dyspepsia have PUD (positive likelihood ratio [LR+] = 2.2; negative likelihood ratio [LR−] = 0.45).11 Specific symptoms that increase the likelihood of PUD in patients with dyspepsia include pain relieved by food (LR+ = 3.3; LR− = 0.7), nighttime awakening due to pain that is relieved by food (LR+ = 2.8; LR− = 0.6), and episodic pain (LR+ = 2.3; LR− = 0.3).10
Upper endoscopy provides direct visualization of the upper gastrointestinal tract and the ability to obtain tissue samples to test for H. pylori and malignancy.12,13 The American College of Gastroenterology (ACG) and Canadian Association of Gastroenterology recommend upper endoscopy in patients 60 years and older with new symptoms of dyspepsia or in adults of any age with alarm symptoms suggestive of malignancy or structural disease (e.g., unintended weight loss, overt gastrointestinal bleeding, iron deficiency anemia, worsening odynophagia or dysphagia, recurrent vomiting, a palpable mass, lymphadenopathy).2 Patients younger than 60 years without alarm symptoms should be tested for H. pylori and treated to eradicate the infection if the result is positive (test and treat).2
How Is H. pylori Infection Diagnosed?
The test-and-treat strategy for H. pylori infection is the mainstay of outpatient management in symptomatic patients who are younger than 60 years, do not have alarm symptoms, and are at low risk of gastric cancer.13 The urea breath test and stool antigen test are the most accurate noninvasive H. pylori tests.
EVIDENCE SUMMARY
Table 1 outlines the testing methods to detect H. pylori.14–18 Noninvasive testing methods are preferred in patients not requiring endoscopy. The urea breath test requires the ingestion of urea labeled with the nonradioactive isotope carbon 13 and has a high specificity and sensitivity.15–17 The stool antigen test using monoclonal antibodies detects H. pylori antigens and has similar accuracy as the breath test.15,17,18 Both tests can also be used to monitor treatment effectiveness. Serologic antibody testing detects immunoglobulin G to H. pylori. However, this test cannot distinguish between active and past infection and cannot be used to monitor treatment effectiveness or verify resolution.15,17 Invasive testing includes upper endoscopy and tissue biopsy, which have high specificity for detecting H. pylori associated with peptic ulcers.14,19
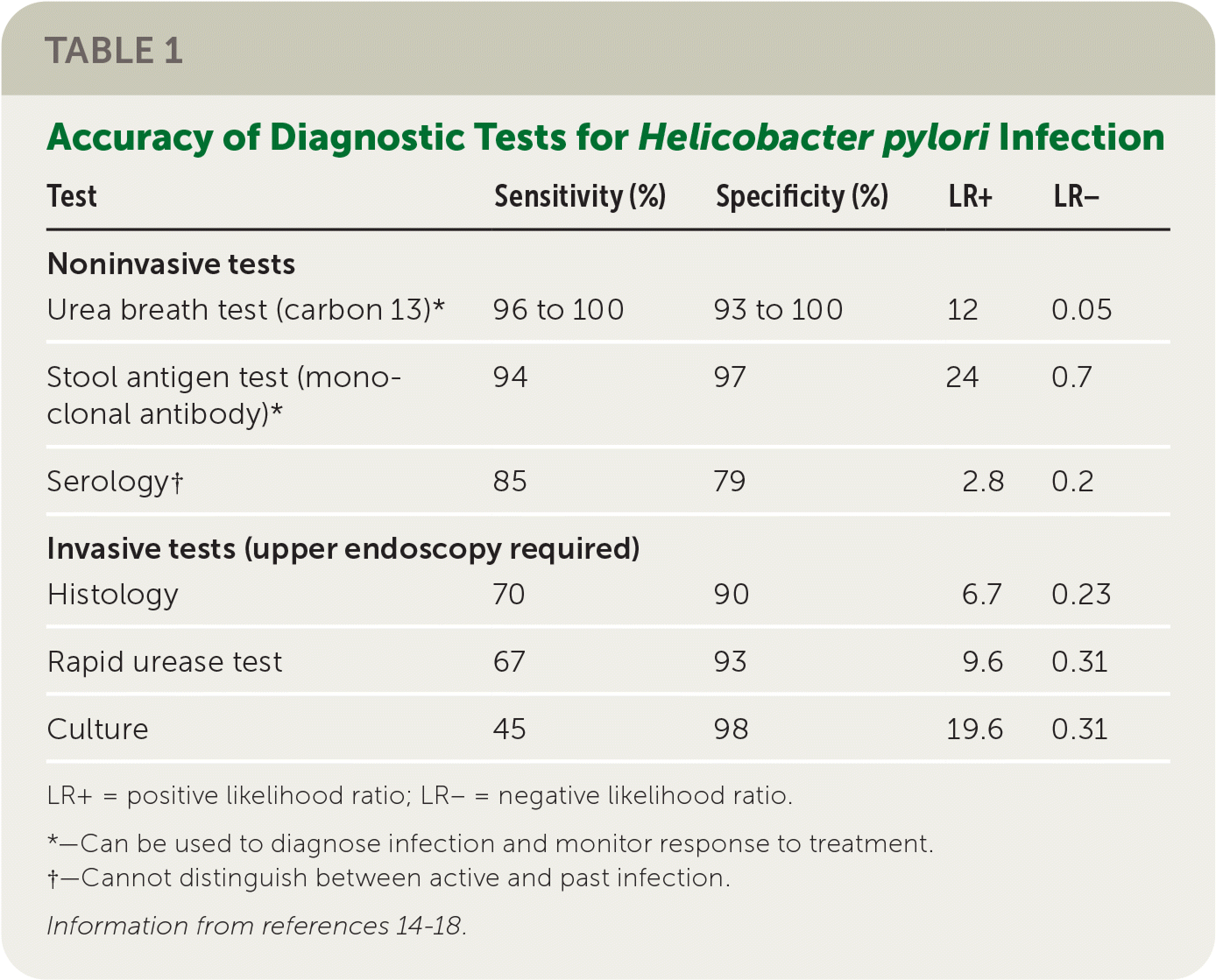
The accuracy of some tests can be influenced by recent use of antibiotics and proton pump inhibitors (PPIs). Antibiotics should be discontinued for four weeks and PPIs for two weeks before urea breath testing, stool antigen testing, endoscopy, and biopsy.15–18 In contrast, accuracy of serology is not affected by use of antibiotics or PPIs.15,17
How Is H. pylori Infection Treated?
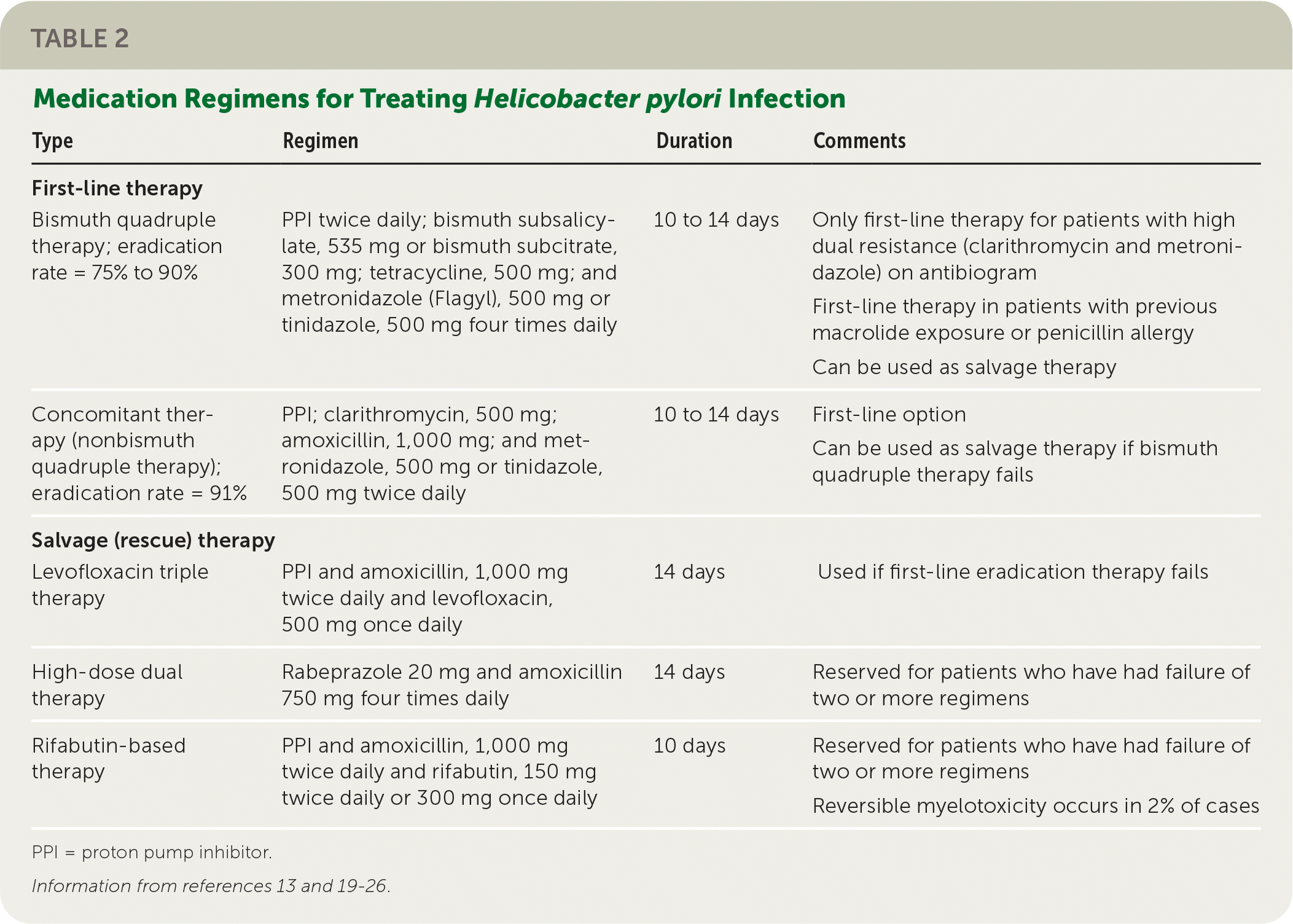
| Type | Regimen | Duration | Comments |
|---|---|---|---|
| First-line therapy | |||
| Bismuth quadruple therapy; eradication rate = 75% to 90% | PPI twice daily; bismuth subsalicylate, 535 mg or bismuth subcitrate, 300 mg; tetracycline, 500 mg; and metronidazole (Flagyl), 500 mg or tinidazole, 500 mg four times daily | 10 to 14 days | Only first-line therapy for patients with high dual resistance (clarithromycin and metronidazole) on antibiogram First-line therapy in patients with previous macrolide exposure or penicillin allergy Can be used as salvage therapy |
| Concomitant therapy (nonbismuth quadruple therapy); eradication rate = 91% | PPI; clarithromycin, 500 mg; amoxicillin, 1,000 mg; and metronidazole, 500 mg or tinidazole, 500 mg twice daily | 10 to 14 days | First-line option Can be used as salvage therapy if bismuth quadruple therapy fails |
| Salvage (rescue) therapy | |||
| Levofloxacin triple therapy | PPI and amoxicillin, 1,000 mg twice daily and levofloxacin, 500 mg once daily | 14 days | Used if first-line eradication therapy fails |
| High-dose dual therapy | Rabeprazole 20 mg and amoxicillin 750 mg four times daily | 14 days | Reserved for patients who have had failure of two or more regimens |
| Rifabutin-based therapy | PPI and amoxicillin, 1,000 mg twice daily and rifabutin, 150 mg twice daily or 300 mg once daily | 10 days | Reserved for patients who have had failure of two or more regimens Reversible myelotoxicity occurs in 2% of cases |
EVIDENCE SUMMARY
The effectiveness of the once standard triple therapy, composed of a PPI, clarithromycin, and amoxicillin or metronidazole (Flagyl), has waned because of increasing worldwide H. pylori resistance to that regimen.20,27 Although guidelines from all three major consensus groups recommend basing treatment regimens on antibiotic susceptibility data, these data are sparse in the U.S. population.13,19,22
Bismuth quadruple therapy, composed of a PPI, bismuth, tetracycline, and metronidazole or tinidazole, is the recommended first-line treatment in guidelines from the ACG, Maastricht V/Florence Consensus, and Toronto Consensus because this regimen is not affected by increasing clarithromycin resistance.13,19,22 The average intent-to-treat eradication rate of quadruple therapy is 85%; however, adverse effects in 47% of patients, the large number of pills required, and the four-times-daily dosing likely reduce patient adherence.13,23,24
Concomitant therapy (also called nonbismuth quadruple therapy) is another effective option wherein a PPI and three antibiotics (clarithromycin, amoxicillin, and metronidazole or tinidazole) are given concomitantly. The 91% average intent-to-treat eradication rate with concomitant therapy is similar to that of bismuth quadruple therapy.23,24
Sequential therapy and hybrid therapy have eradication rates of less than 90% and can be affected by resistance to clarithromycin. Therefore, these therapies are not recommended by the Maastricht V/Florence Consensus or the Toronto Consensus, although the ACG conditionally recommends them.19,20,22,24
If first-line eradication therapy fails, all three consensus groups recommend salvage (rescue) therapy with bismuth quadruple therapy or levofloxacin triple therapy, if not previously used.13,19,20,22 Clarithromycin-based or levofloxacin regimens should be avoided if used previously. Metronidazole can be reused in a regimen if bismuth is added, or it can be replaced with tinidazole regardless of bismuth use.13,19,20,22 If multiple attempts at eradication fail, high-dose dual therapy or rifabutin-based therapy should be considered.24,26
A test of cure is recommended using the urea breath test or stool antigen test four weeks after completion of therapy and at least two weeks after stopping PPIs in those with persistent dyspepsia or a known ulcer, mucosal-associated lymphoid tissue tumor, or gastric cancer.28
How Is PUD Treated?
EVIDENCE SUMMARY
In those who are H. pylori negative, there is no difference in rates of persistent symptoms between empiric PPI therapy and prompt endoscopy.2 First-line treatment for PUD consists of PPI therapy for at least eight weeks.29 Although histamine H2 blockers are sometimes prescribed, ulcer-healing rates with PPIs are almost double those with H2 blockers.29
How Can NSAID-Induced PUD and Its Complications Be Prevented?
Risk factors for NSAID-induced PUD include older age, previous ulcer, and the use of aspirin or other NSAIDs, anticoagulants, or corticosteroids.29,30 Therapeutic strategies aimed at reducing this risk in patients who continue to use NSAIDs include coadministering a PPI, H2blocker, or misoprostol; substituting a cyclooxygenase-2 (COX-2) inhibitor for the NSAID; and test and treat for H. pylori. 29–33
EVIDENCE SUMMARY
Gastroprotective therapies are helpful in the primary and secondary prevention of PUD in high-risk individuals. Patients at high risk of developing PUD who are unable to stop NSAIDs should be treated with a PPI. A meta-analysis showed that prescribing any gastroprotective therapy decreased the likelihood of an endoscopically diagnosed ulcer with a number needed to treat of 7 over six months.33 Substituting a COX-2 inhibitor such as celecoxib for a nonselective NSAID decreases the incidence of gastric and duodenal ulcers (number needed to treat = 12 over six months).34 A Cochrane review suggests that high-risk patients should take a COX-2 inhibitor and a PPI concurrently for the best protection.32 For patients at high risk of cardiovascular events, naproxen is the safest NSAID unless gastrointestinal risk is also high. Table 3 includes ACG and Canadian Association of Gastroenterology recommendations for NSAID therapy based on cardiovascular and gastrointestinal risk.31,34
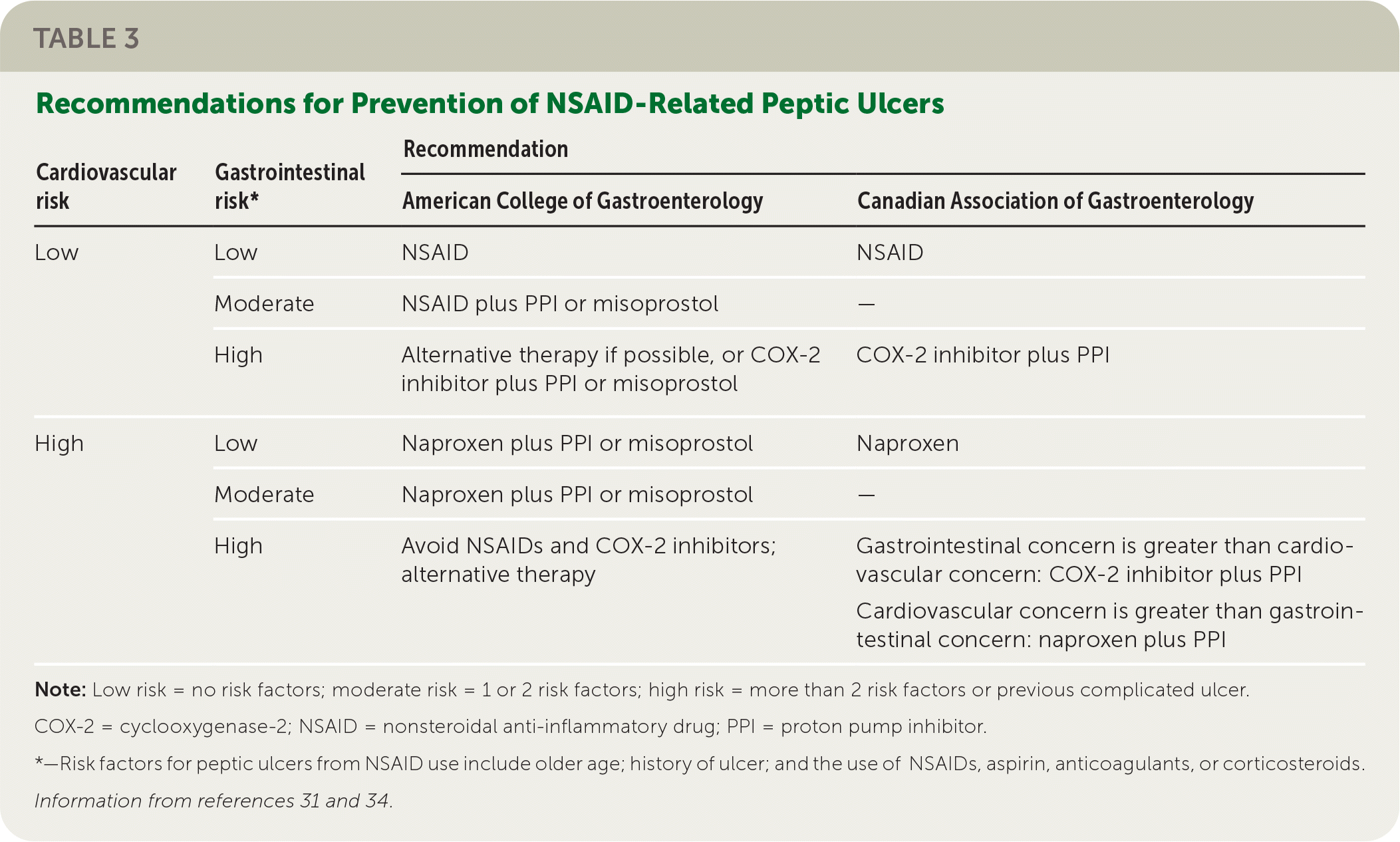
| Cardiovascular risk | Gastrointestinal risk* | Recommendation | |
|---|---|---|---|
| American College of Gastroenterology | Canadian Association of Gastroenterology | ||
| Low | Low | NSAID | NSAID |
| Moderate | NSAID plus PPI or misoprostol | — | |
| High | Alternative therapy if possible, or COX-2 inhibitor plus PPI or misoprostol | COX-2 inhibitor plus PPI | |
| High | Low | Naproxen plus PPI or misoprostol | Naproxen |
| Moderate | Naproxen plus PPI or misoprostol | — | |
| High | Avoid NSAIDs and COX-2 inhibitors; alternative therapy | Gastrointestinal concern is greater than cardiovascular concern: COX-2 inhibitor plus PPI Cardiovascular concern is greater than gastrointestinal concern: naproxen plus PPI | |
Among patients who are positive for H. pylori, those taking NSAIDs are more likely to develop peptic ulcers (OR = 1.81; 95% CI, 1.40 to 2.36) and bleeding ulcers (OR = 5.21; 95% CI, 3.48 to 7.78).35 Although coprescribing a maintenance PPI is effective for preventing NSAID-related ulcers, eradicating H. pylori in NSAID users is more effective (OR = 0.50; 95% CI, 0.36 to 0.74) and should be offered if the patient is positive.31,36,37
Are There Risks to Long-Term PPI Use, and Can PPIs Be Deprescribed in Patients With Well-Controlled Symptoms?
There are several potential risks associated with long-term PPI use, including fractures, chronic kidney disease, Clostridioides difficile infection, dementia, gastric cancer, and micronutrient deficiencies, although evidence for many of these factors is limited (eTable A).

| Potential risk | Evidence summary |
|---|---|
| Bone fractures | In 2011, the FDA retracted the 2010 boxed warning about possible increased fracture risk with long-term PPI use because of insufficient evidence.A1 There is insufficient evidence to recommend changes to bone mineral density monitoring with PPI use.A1 |
| Cardiovascular events when taken with the antiplatelet agent clopidogrel | The FDA discourages coprescribing omeprazole with clopidogrel because of a potential reduction in antiplatelet activity. Omeprazole inhibits hepatic enzyme cytochrome P450 2C19, which metabolizes clopidogrel to active metabolites. A large randomized controlled trial found no increased risk of cardiovascular events in patients who took a PPI and clopidogrel together.A2 A meta-analysis of smaller studies suggested an increased risk of cardiovascular events.A3 Because reduction in bleeding complications outweighs the risk of cardiovascular events in patients with high risk of bleeding, pantoprazole is recommended to reduce bleeding risk.A4 |
| Chronic kidney disease | Two studies suggest that there may be a higher incidence of CKD in those taking PPIs. One prospective cohort study showed an increased risk of acute kidney injury and CKD (hazard ratio = 1.45; 95% CI, 1.11 to 1.99), and one meta-analysis demonstrated an increased risk of CKD and end-stage renal disease (relative risk = 1.33; 95% CI, 1.18 to 1.51).A5,A6 |
| Clostridioides difficile infections | Although there is an increased incidence of C. difficile infections in hospitalized patients taking PPIs, there are no data in the community setting.A7,A8 |
| Dementia | Two German observational studies found a 38% to 44% increased risk of dementia in patients taking PPIs.A9,A10 The mechanism of this association and the contribution of factors such as age and comorbidities are unknown. |
| Gastric cancer | Long-term PPI use slightly increases the risk of gastric cancer compared with histamine H2 blockers (number needed to harm = 2,121 over 5 years and 1,191 over 10 years).A11 |
| Micronutrient deficiency | Long-term PPI use may decrease levels of magnesium, calcium, and vitamin B12, although the clinical significance of these changes has not been defined.A12,A13 |
EVIDENCE SUMMARY
Effects on osteoporotic fracture risk are uncertain.38 The risk of acute coronary syndrome appears to be limited to those taking omeprazole with clopidogrel; if a PPI is needed, pantoprazole is preferred.39–41 There may be an increased risk of chronic kidney disease.42,43 Elevated risk of C. difficile infection with PPI use has been demonstrated only in hospitalized patients.44,45 There is a slightly increased risk of gastric cancer with long-term use of PPIs compared with H2 blockers, with a number needed to harm of 1,191 over 10 years.46
A Canadian guideline recommends deprescribing PPIs for patients who have completed at least a four-week course of a PPI and had resolution of symptoms. Deprescribing can be completed by reducing the dose, stopping the drug, or using it only when needed.47 This recommendation is based on a systematic review of deprescribing that showed a low risk of symptom relapse. Deprescribing of PPIs is not recommended for patients with Barrett esophagus or a documented history of a bleeding ulcer or severe esophagitis or for patients taking long-term NSAIDs who have increased bleeding risk.47
What Are Considerations for Special Populations?
OLDER PATIENTS
Older people have increased risk of PUD because they more commonly use NSAIDs and aspirin.48 In addition, when PUD is present, they less often report abdominal pain and other symptoms, making detection difficult.49 Clinicians should consider reducing or discontinuing NSAIDs if possible in this population. If NSAID use cannot be stopped, consideration should be given to coprescribing a PPI to reduce the risk of PUD.47 Testing for H. pylori infection should be considered in adults who are symptomatic, with treatment if present.50 Clarithromycin and fluoroquinolone dosing may need to be adjusted in older patients who have chronic kidney disease or take warfarin.51
CHILDREN
Children with PUD may present with iron deficiency anemia or the nonspecific symptom of nausea or vomiting and often do not report typical epigastric abdominal pain.52 Urea breath testing for H. pylori infection should be considered, with eradication treatment if present to reduce the risk of gastric cancer in adulthood.52 Upper endoscopy is usually not needed in children unless ulcer disease or esophagitis is suspected.
PREGNANT PATIENTS
Dyspepsia is common in pregnancy and often related to pregnancy-associated gastroesophageal reflux disease; however, PUD also occurs in pregnancy. Upper endoscopy is usually avoided in pregnancy to reduce the risk of complications for mother and fetus.53 Pregnant women who have H. pylori infection with mild symptoms can postpone treatment until after delivery and the completion of breast-feeding. If more severe symptoms are present, including hyperemesis gravidarum, clinicians should consider seven days of treatment with a PPI, amoxicillin, and metronidazole in the first trimester.50,52 Clarithromycin can be used instead of metronidazole in the second and third trimesters and while breastfeeding.51,53 Fetal exposure to PPIs during pregnancy does not increase the risk of congenital malformations or infant morbidity or mortality.54
This article updates previous articles on this topic by Fashner and Gitu55; Ramakrishnan and Salinas3; Ables, et al.56; and Meurer and Bower.57
Data Sources: A search was completed using the key words peptic ulcer disease, H. pylori, risk, prevention, dyspepsia, gastric cancer, gastritis, ulcer, test and treat, PPI, eradication, and gastroprotective clinical decision rule. The search included the Agency for Healthcare Research and Quality Evidence Reports, Cochrane Database of Systematic Reviews, Essential Evidence Plus, and PubMed Clinical Queries. Search dates: January 2022 and December 2022.
