
Am Fam Physician. 2023;107(2):159-164
Author disclosure: No relevant financial relationships.
Trigger points producing myofascial pain syndromes are common in primary care. Located within skeletal muscle, trigger points are taut, band-like nodules capable of producing pain and disability. Some evidence from clinical trials supports massage, physical therapy, and osteopathic manual medicine as first-line less invasive treatment strategies. Trigger points are often treated with injections; although randomized trials have found statistically significant results with trigger point injections, conclusions are limited by low numbers of study participants, difficulty in blinding, the potential for a placebo effect, and lack of posttreatment follow-up. No single pharmacologic agent used in trigger point injections has been proven superior to another, nor has any single agent been proven superior to placebo. Trigger point injections, therefore, should be reserved for patients whose myofascial pain has been refractory to other measures, and family physicians should first employ less invasive treatment strategies. Trigger point management is only one part of a comprehensive, multimodal, and team-based approach to patients with myofascial pain.
| Clinical recommendation | Evidence rating | Comments |
|---|---|---|
| Placebo effect may be the underlying source of pain relief from trigger point injections.9–11 | B | A strong placebo-type effect is seen in a systematic review of numerous randomized controlled trials in which a painful intervention (i.e., trigger point injections) is introduced to a painful condition (trigger point), producing results with placebo-type effect |
| Massage and physical therapy should be considered as first-line less invasive treatments for trigger point pain.9,16 | B | Systematic review of low-quality evidence shows effective trigger point management with less invasive methods; when added risk of patient harm is introduced, less invasive therapies are considered more reasonable |
| Routine use of trigger point injections is not supported by clinical trials.9,10,12,13,16 | B | Trigger point injection trials have methodologic flaws, small numbers of trial participants, difficulty blinding, and lack of long-term follow-up; a systematic review of low-quality randomized controlled trials consistently demonstrates this pattern |
A study in a primary care practice found that 30% of patients presenting with musculoskeletal pain also had myofascial pain.4 Located anywhere skeletal muscle is found, trigger points most commonly occur over the muscles of the back.2,5 In studies of patients presenting with myofascial pain, the majority of all trigger points came from the trapezius muscle. Typical locations for trapezius trigger points are pictured in Figure 1.2,6 Gluteus maximus, gluteus medius, and quadratus lumborum muscles also commonly harbor trigger points. Areas of the back that tend to be affected in patients presenting with trigger points are demonstrated in Figure 2.3
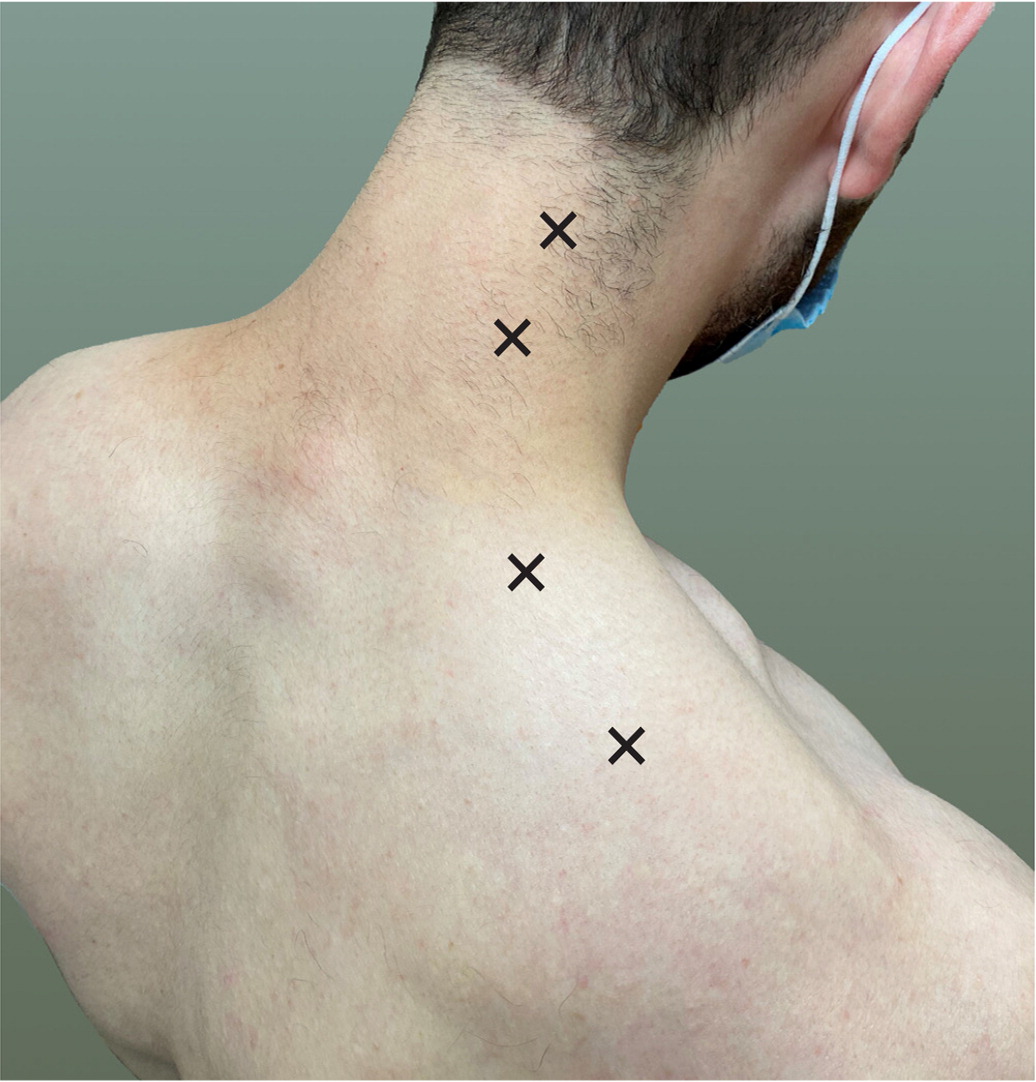
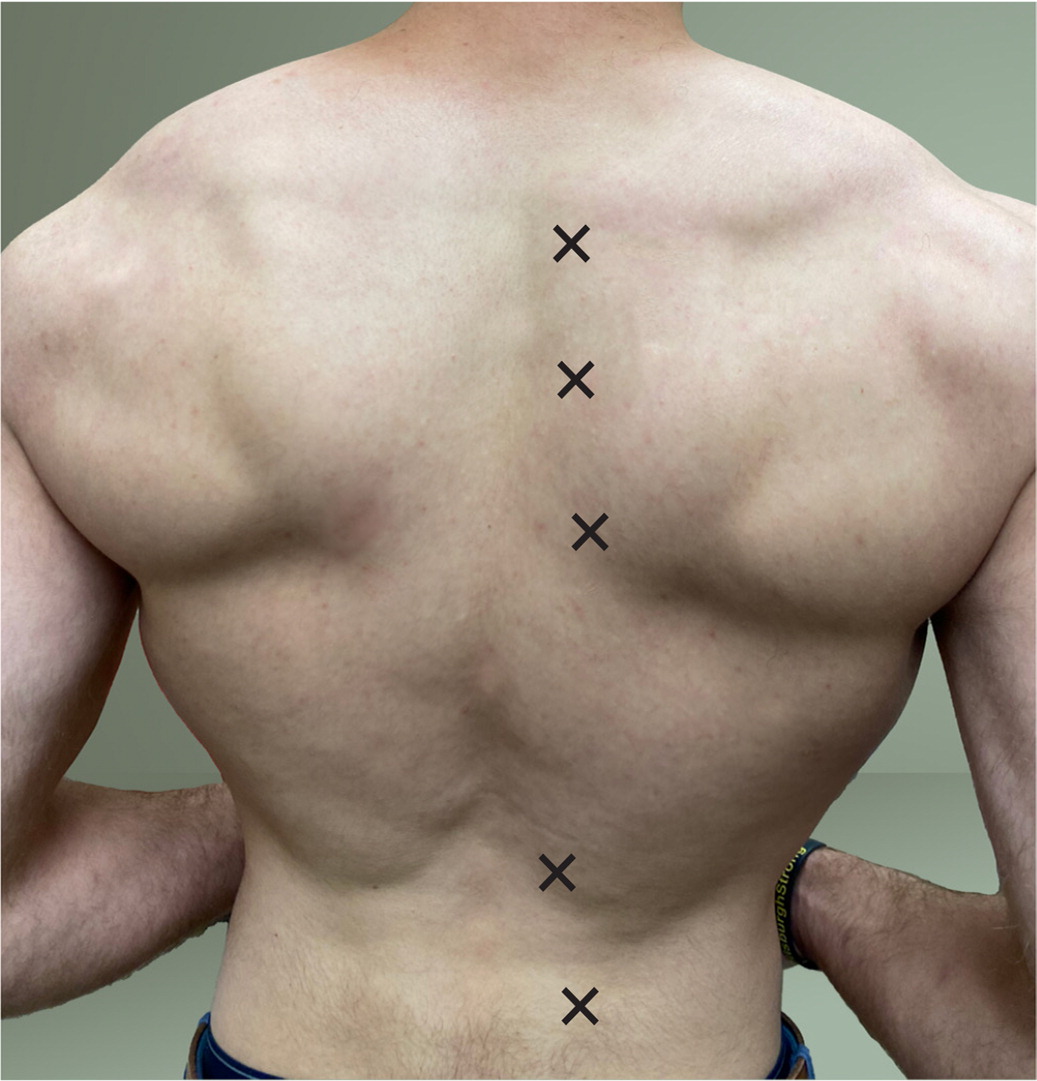
Diagnosis
In patients with musculoskeletal pain, especially in the neck or back, identifying trigger points should be part of a detailed history and neuromuscular examination. Physical examination is key to determining the location and number of myofascial trigger points. During the palpation portion of the examination, trigger points are elicited when the patient experiences pain while the physician palpates a band-like nodule within the muscle.2
Less Invasive Management of Trigger Points
Nonpharmacologic treatment modalities for trigger points have been studied, but no standardized treatment protocol has been established.2 Trigger point treatments include oral nonsteroidal anti-inflammatory drugs, acetaminophen, and muscle relaxants. Evidence for the use of medications in trigger point management is lacking.7 Other less invasive treatments include massage, osteopathic manual medicine, physical therapy (PT), and the spray and stretch technique. Proposed invasive strategies include acupuncture, dry needling, and trigger point injections using pharmacologic agents.
Although some statistically significant benefits have been noted in some randomized trials of trigger point therapy, the results are at high risk of bias. Studies have typically had small sample sizes, with difficulty blinding patients to the interventions. Studies of injection therapies have differences in injection techniques and variation in needle sizes. The benefits observed using different injection compositions (including normal saline) suggest a strong placebo response to trigger point injection.8 The underlying source of pain relief from trigger point injections may be the placebo effect.9–11 The absence of posttreatment patient follow-up in randomized controlled trials (RCTs) of trigger point management also hinders drawing conclusions about long-term clinical effects, especially for trigger point injections.2,8,12–14
MASSAGE
Massage is a modality in which direct pressure is applied in a slow, controlled fashion over the trigger point.1,15 One recent RCT involved 56 patients with tension-type headaches who were randomized to receive 12 massage treatments or sham treatment (detuned ultrasonography) or to be wait-listed over a six-week period.9 Outcomes for the trial included self-reported headache pain and pain-pressure threshold measured with an algometer (a device measuring pressure against musculature). No differences were found between the massage and placebo groups in headache frequency; however, the pain-pressure threshold and self-reported headache pain improved in the massage-treated patients. A similar RCT containing 62 patients with tension-type headache compared massage with sham massage over 12 sessions.10 A statistically significant improvement in pain-pressure threshold was observed over the trapezius and suboccipital muscles in the massage-treated group, representing an increased ability to tolerate pain. Outcome measures commonly used in trigger point management trials are summarized in eTable A.

| Outcome measure | Definition |
|---|---|
| Likert scale | Respondents specify level of agreement-disagreement on a symmetrical scale for a series of statements |
| Neck Disability Index https://www.smcnd.org/assets/docs/pt/neck_disability_index.pdf | A 10-item questionnaire measuring self-reported pain and perceived disability determined using activities of daily living |
| Pain Numeric Rating Scale https://www.va.gov/painmanagement/docs/pain_numberic_rating_scale.pdf | An 11-point self-reported pain scale focusing on function and activities of daily living |
| Pain-pressure threshold | A handheld algometer used to apply gradually increasing pressure over muscle tissue in assessment of muscular sensitivity |
| Verbal rating scale | Self-reported descriptors of pain using a standardized set of words or phrases (e.g., mild, moderate, severe) |
| Visual analog scale | Using questionnaires, respondents specify their pain level |
PHYSICAL THERAPY
PT has also been studied as a tool for trigger point management.9,12 A recent meta-analysis of 24 RCTs using PT to manage trigger points showed beneficial effects.16 These PT programs improved pain-pressure thresholds, reduced pain intensity, and improved range of motion, but they showed no effect on improving disability in patients with trigger points. Effect sizes were moderate for outcomes such as pain intensity, pain-pressure threshold, and range of motion.16 One RCT involving 66 breast cancer survivors in a supervised PT program that used swimming pool exercises demonstrated a statistically significant reduction in the number of trigger points located in upper body musculature over eight weeks compared with a control group who received no PT.12 A statistically significant reduction in scalene muscle trigger points was measured on the breast cancer–affected side as well as on the unaffected side. The subjective outcomes measured within these trials suggest possible clinical utility for PT in trigger point management.16
SPRAY AND STRETCH TECHNIQUE
The spray and stretch technique involves stretching the skin over a trigger point and applying a topical anesthetic spray, such as ethyl chloride. A topical anesthetic introduces a sudden sensory stimulus that is thought to distract the patient from the discomfort within the trigger point–affected muscle.17 However, no good-quality RCTs have evaluated clinical benefit of the spray and stretch technique.
OSTEOPATHIC MANUAL MEDICINE
Muscle energy and counter-strain techniques are used in the treatment of trigger points in osteopathic medicine and by physical therapists. The muscle energy technique involves voluntary muscle contraction by the patient in a direction against a force provided by the physician for three to five seconds, producing isometric contraction. Following a post-isometric relaxation phase, the muscle is then stretched to a new point of restriction; this is repeated for three to five cycles.18 Counter strain is a manual technique involving the manipulation of joints and muscles away from a restrictive barrier toward a position of ease until trigger point pain is reduced. This position is then maintained for 90 seconds before returning to a neutral position.18 A recent single-blind RCT using osteopathic manual medicine to treat piriformis syndrome trigger points randomized 48 patients to receive integrated neuromuscular treatment (i.e., a combination of counter-strain and positional release techniques) in one group or positional release technique alone in a second group over a period of eight weeks.19 Visual analog scales and self-reported quality-of-life outcome measures demonstrated superiority of integrated neuromuscular treatment over positional release technique in all outcomes measured.19 This single-blind RCT demonstrated clinically significant improvement in alleviating trigger point pain immediately and at four-month follow-up.
Trigger Point Injections
Trigger point injections use pharmacologic agents such as lidocaine, corticosteroids (e.g., methylprednisolone, triamcinolone acetonide), hyaluronidase (Amphadase), onabotulinumtoxinA, sodium bicarbonate, and ozone, as well as normal saline or sterile water.20 No single pharmacologic agent or mixture of active drugs has been proven superior to another in the treatment of trigger points, nor has any agent been proven superior to placebo.2,21
Trigger point injections have been studied in muscles of the face, occiput, neck, shoulders, and extremities.22 See a video demonstrating a trigger point injection for musculoskeletal neck pain. Trigger point injection has also been employed in the treatment of temporomandibular joint disorders, pelvic floor pain, and headaches. However, studies have been small and have, at best, been found to be inconsistent and/or more commonly have no clear evidence of benefit.11,23,24
Trials using trigger point injections are summarized in Table 1.2,9,11,14,25,26 For example, one recent trial randomized 48 patients presenting to the emergency department with trigger points to receive normal saline or a mixture of lidocaine 1% and triamcinolone acetonide, 40 mg.2 Following injection, no differences were observed in pain scores at the time of emergency department discharge, nor at two weeks using numeric rating scales. Thus, normal saline was found to be noninferior to the injected drug mixture. This is an example of a strong placebo-type effect that is commonly observed in trigger point injection trials. Adverse effects included headache, nausea, and postinjection soreness.
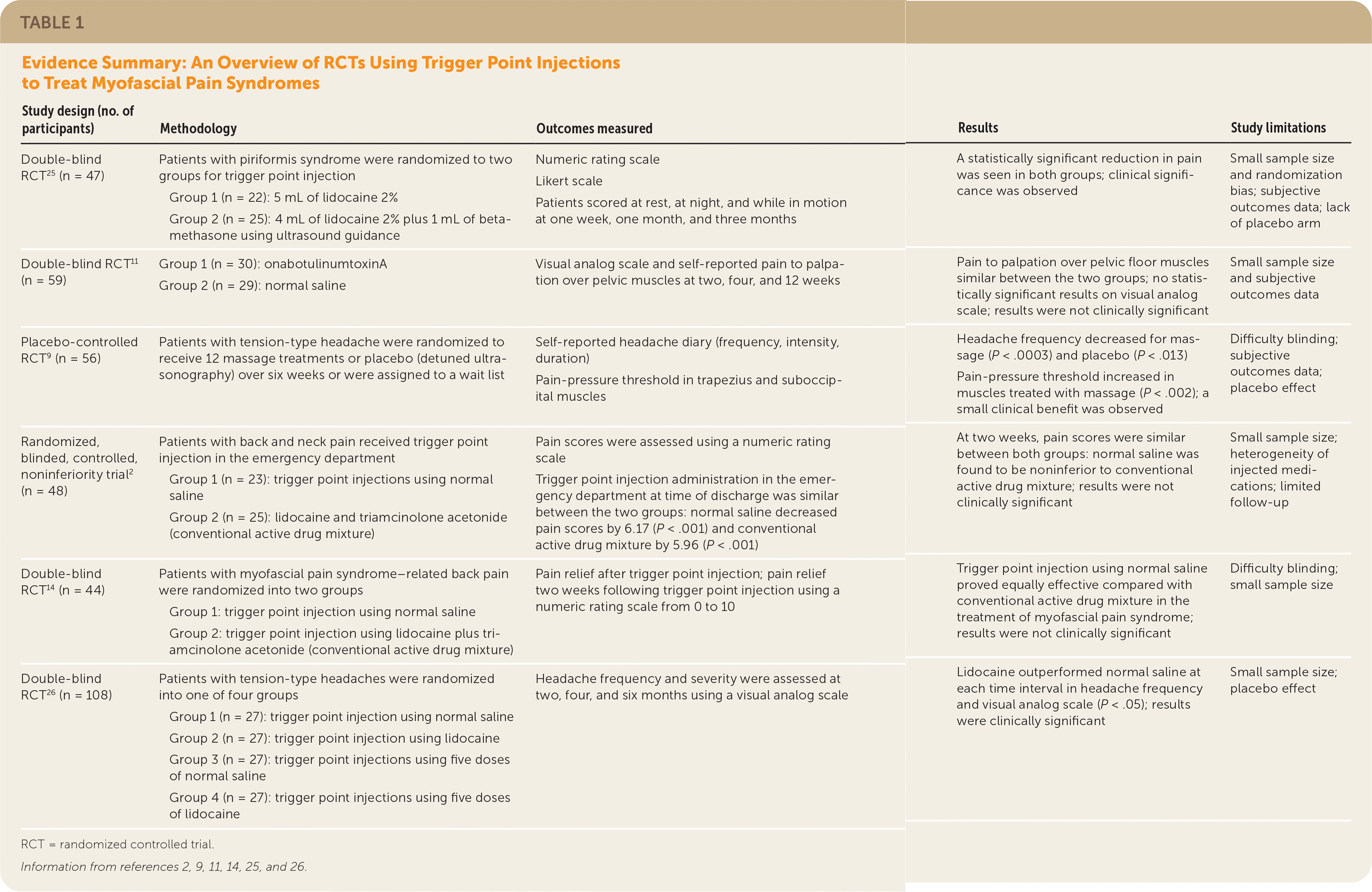
| Study design (no. of participants) | Methodology | Outcomes measured | Results | Study limitations |
|---|---|---|---|---|
| Double-blind RCT 25 (n = 47) | Patients with piriformis syndrome were randomized to two groups for trigger point injection Group 1 (n = 22): 5 mL of lidocaine 2% Group 2 (n = 25): 4 mL of lidocaine 2% plus 1 mL of betamethasone using ultrasound guidance | Numeric rating scale Likert scale Patients scored at rest, at night, and while in motion at one week, one month, and three months | A statistically significant reduction in pain was seen in both groups; clinical significance was observed | Small sample size and randomization bias; subjective outcomes data; lack of placebo arm |
| Double-blind RCT 11 (n = 59) | Group 1 (n = 30): onabotulinumtoxinA Group 2 (n = 29): normal saline | Visual analog scale and self-reported pain to palpation over pelvic muscles at two, four, and 12 weeks | Pain to palpation over pelvic floor muscles similar between the two groups; no statistically significant results on visual analog scale; results were not clinically significant | Small sample size and subjective outcomes data |
| Placebo-controlled RCT9 (n = 56) | Patients with tension-type headache were randomized to receive 12 massage treatments or placebo (detuned ultrasonography) over six weeks or were assigned to a wait list | Self-reported headache diary (frequency, intensity, duration) Pain-pressure threshold in trapezius and suboccipital muscles | Headache frequency decreased for massage (P < .0003) and placebo (P < .013) Pain-pressure threshold increased in muscles treated with massage (P < .002); a small clinical benefit was observed | Difficulty blinding; subjective outcomesdata; placebo effect |
| Randomized, blinded, controlled, noninferiority trial2 (n = 48) | Patients with back and neck pain received trigger point injection in the emergency department Group 1 (n = 23): trigger point injections using normal saline Group 2 (n = 25): lidocaine and triamcinolone acetonide (conventional active drug mixture) | Pain scores were assessed using a numeric rating scale Trigger point injection administration in the emergency department at time of discharge was similar between the two groups: normal saline decreased pain scores by 6.17 (P < .001) and conventional active drug mixture by 5.96 (P < .001) | At two weeks, pain scores were similar between both groups: normal saline was found to be noninferior to conventional active drug mixture; results were not clinically significant | Small sample size; heterogeneity of injected medications; limited follow-up |
| Double-blind RCT 14 (n = 44) | Patients with myofascial pain syndrome–related back pain were randomized into two groups Group 1: trigger point injection using normal saline Group 2: trigger point injection using lidocaine plus triamcinolone acetonide (conventional active drug mixture) | Pain relief after trigger point injection; pain relief two weeks following trigger point injection using a numeric rating scale from 0 to 10 | Trigger point injection using normal saline proved equally effective compared with conventional active drug mixture in the treatment of myofascial pain syndrome; results were not clinically significant | Difficulty blinding; small sample size |
| Double-blind RCT 26 (n = 108) | Patients with tension-type headaches were randomized into one of four groups Group 1 (n = 27): trigger point injection using normal saline Group 2 (n = 27): trigger point injection using lidocaine Group 3 (n = 27): trigger point injections using five doses of normal saline Group 4 (n = 27): trigger point injections using five doses of lidocaine | Headache frequency and severity were assessed at two, four, and six months using a visual analog scale | Lidocaine outperformed normal saline at each time interval in headache frequency and visual analog scale (P < .05); results were clinically significant | Small sample size; placebo effect |
DRY NEEDLING
Dry needling involves a single puncture of the skin overlying a trigger point or repeated redirection of a needle within a trigger point without the use of injected medication. In a recent RCT, 168 patients with chronic tension-type headache were randomized to receive dry needling or sham dry needling delivered in three sessions over two weeks.27 The sham dry needling protocol involved dry needling into adipose tissue in an area where an active trigger point was absent. The primary outcome was headache intensity with secondary outcomes of headache frequency, duration, and self-reported quality of life. At one-month follow-up, statistically and clinically significant improvement occurred in all measures within the dry needling group compared with the sham group (P < .05). However, differences in injection techniques and lack of long-term follow-up for these patients produced trial results with questionable clinical utility.27 Similar to RCTs involving acupuncture, RCTs examining dry needling in the treatment of trigger points have difficulty blinding and are at high risk of bias, demonstrating a placebo-type effect.
Complications of trigger point injection and dry needling are rare; however, serious injuries have occurred, including pneumothorax and spinal cord injury. Contraindications to and complications of trigger point injection therapy are summarized in Table 2.2,28–32 Because of reported adverse effects and the potential for patient harm, and because the evidence for trigger point injections and dry needling in most studies has been similar to placebo, less invasive methods are used as first-line treatments for trigger point management (e.g., massage, PT, manual manipulation, spray and stretch technique). Routine use of trigger point injections as initial therapy is not supported by clinical trials.9,10,12,13,16 Invasive trigger point management techniques should be reserved for patients in whom other measures for myofascial pain control have failed. However, patients may request invasive interventions for myofascial trigger point pain, and a shared decision-making process is recommended.
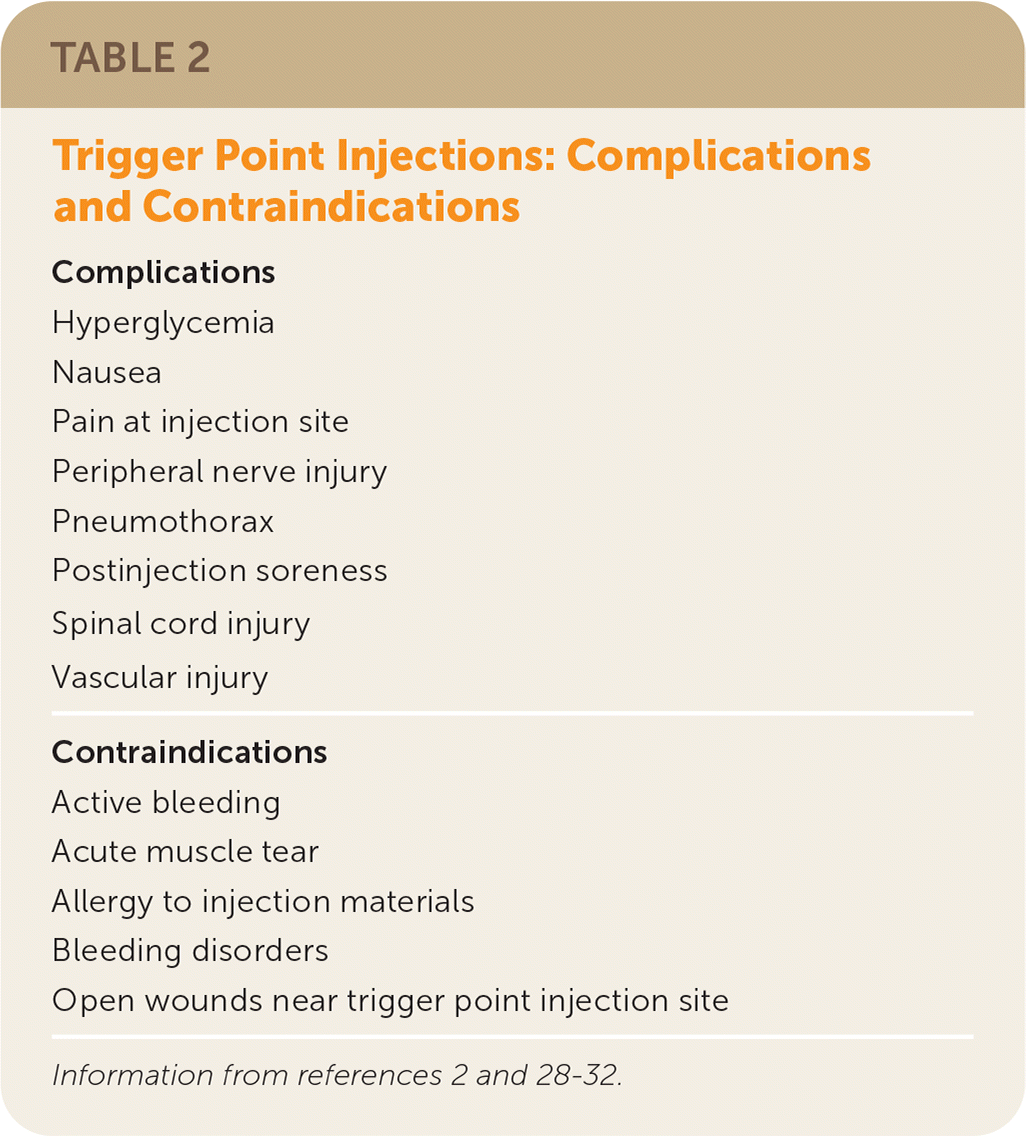
| Complications |
| Hyperglycemia |
| Nausea |
| Pain at injection site |
| Peripheral nerve injury |
| Pneumothorax |
| Postinjection soreness |
| Spinal cord injury |
| Vascular injury |
| Contraindications |
| Active bleeding |
| Acute muscle tear |
| Allergy to injection materials |
| Bleeding disorders |
| Open wounds near trigger point injection site |
This article updates a previous article on this topic by Alvarez and Rockwell.33
Data Sources: The authors searched PubMed, the Cochrane database, and Essential Evidence Plus using keywords trigger point, trigger point injections, local anesthesia, myofascial pain syndromes, and fibromyalgia. The search included randomized controlled trials, clinical trials, meta-analyses, and reviews. Search dates: May 25, 2021, to December 1, 2021; December 14, 2022.
