Am Fam Physician. 2024;109(6):513-514
Author disclosure: No relevant financial relationships.
Details for This Review
Study Population: 21 randomized controlled trials (RCTs; N = 3,723) performed in 16 countries between 1966 and 2017; women 15 to 33 years of age with primary dysmenor-rhea (at least moderate pain for at least 1 day of menses) who did not have obvious pelvic pathology on physical examination but did have regular ovulatory menstrual cycles (21- to 35-day cycles)
Efficacy End Points: Primary: difference between the groups in pain rating and the number of women experiencing pain relief at the end of treatment; secondary: difference between groups in the number of women requiring additional pain medication (analgesics) and the ratio of women reporting absences from work or school
Harm End Points: Primary: adverse events from treatment (incidence and type of adverse event); secondary: withdrawals from treatment and adverse events
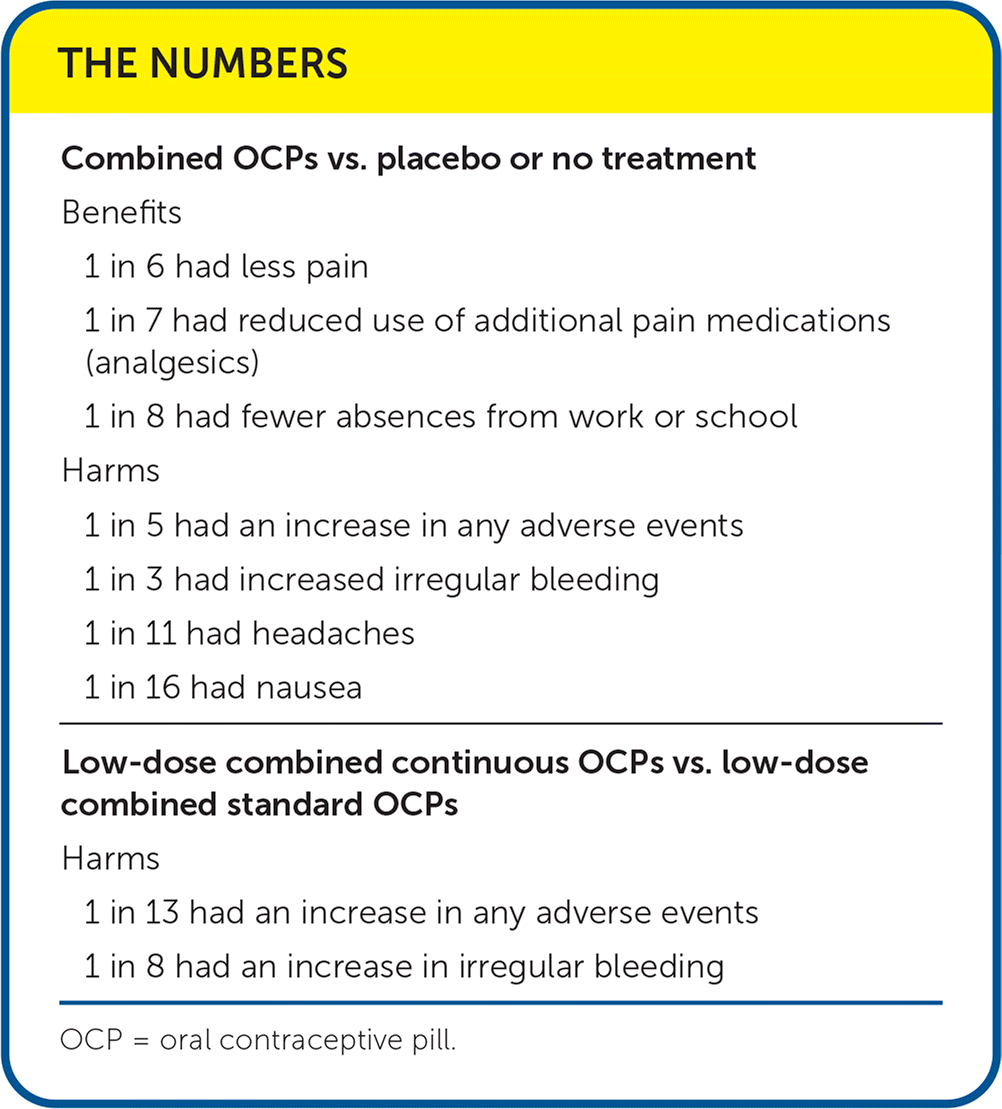
| Combined OCPs vs. placebo or no treatment |
| Benefits |
| 1 in 6 had less pain |
| 1 in 7 had reduced use of additional pain medications (analgesics) |
| 1 in 8 had fewer absences from work or school |
| Harms |
| 1 in 5 had an increase in any adverse events |
| 1 in 3 had increased irregular bleeding |
| 1 in 11 had headaches |
| 1 in 16 had nausea |
| Low-dose combined continuous OCPs vs. low-dose combined standard OCPs |
| Harms |
| 1 in 13 had an increase in any adverse events |
| 1 in 8 had an increase in irregular bleeding |
Subscribe
From $180- Immediate, unlimited access to all AFP content
- More than 125 CME credits/year
- AAFP app access
- Print delivery available
Issue Access
$59.95- Immediate, unlimited access to this issue's content
- CME credits
- AAFP app access
- Print delivery available