
Am Fam Physician. 2025;111(3):210-211
Author disclosure: No relevant financial relationships.
DETAILS FOR THIS REVIEW
Study Population: 27,235 individuals who actively smoke tobacco from 88 studies, including 47 randomized controlled trials (RCTs)
Efficacy End Points: Smoking cessation at least 6 months after the start of the intervention
Harm End Points: Any adverse events or serious adverse events at least 1 week after the start of the intervention
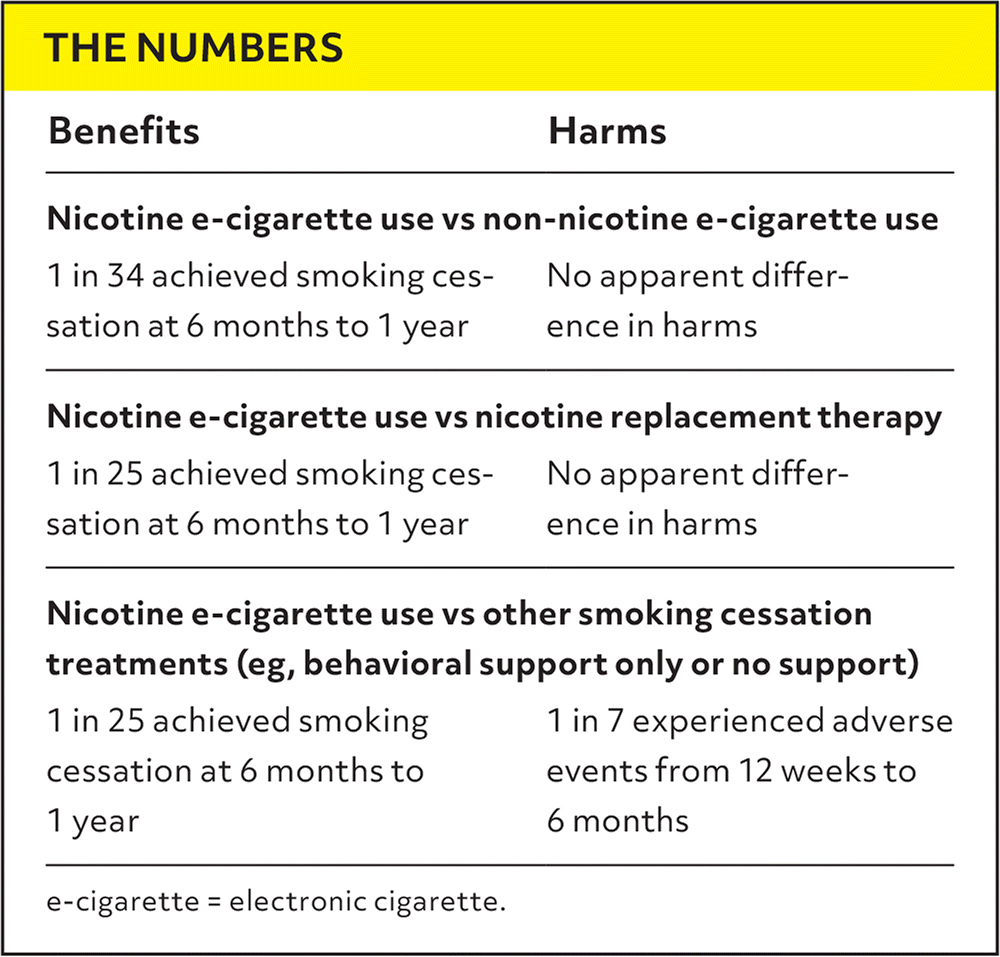
| Benefits | Harms |
|---|---|
| Nicotine e-cigarette use vs non-nicotine e-cigarette use | |
| 1 in 34 achieved smoking cessation at 6 months to 1 year | No apparent difference in harms |
| Nicotine e-cigarette use vs nicotine replacement therapy | |
| 1 in 25 achieved smoking cessation at 6 months to 1 year | No apparent difference in harms |
| Nicotine e-cigarette use vs other smoking cessation treatments (eg, behavioral support only or no support) | |
| 1 in 25 achieved smoking cessation at 6 months to 1 year | 1 in 7 experienced adverse events from 12 weeks to 6 months |
Subscribe
From $180- Immediate, unlimited access to all AFP content
- More than 125 CME credits/year
- AAFP app access
- Print delivery available
Issue Access
$59.95- Immediate, unlimited access to this issue's content
- CME credits
- AAFP app access
- Print delivery available
