
This AAFP educational supplement is supported by an unrestricted grant from GSK.
Fam Pract Manag. 2024;31(3):19-22
Introduction
Adults 60 years and older (particularly those with certain medical conditions) are at increased risk for respiratory syncytial virus (RSV) infection and severe disease and death secondary to RSV.1 Recognizing this risk, the Advisory Committee on Immunization Practices (ACIP) began recommending the RSV vaccine in June 2023 for all adults 60 years and older using shared clinical decision-making (SCDM).
SCDM differs from default recommendations for universal vaccination and entails “the decision to vaccinate an individual patient…based on a discussion between the health care provider and the patient. It may be informed by the patient's risk of severe RSV disease and their characteristics, values, and preferences; the health care provider's clinical discretion; and the characteristics of the vaccine.”2
In addition to RSV, the ACIP recommends the SCDM approach for the following four vaccines and age groups3:
- Meningitis B (16–23 years);
- Human papillomavirus (27–45 years);
- Hepatitis B (60 years and older for patients with diabetes);
- Pneumococcal conjugate vaccine 20 [PCV20] (65 years and older who have completed PCV13 at any age); and
- Pneumococcal polysaccharide 23 (65 years and older).
Due to the added step of SCDM, some physicians and other clinicians may find discussing RSV immunization with patients adds time and complexity to patient visits, which may create a barrier to administering vaccines to patients who benefit from protection against RSV.
This educational supplement highlights the currently available RSV vaccines, identifies patients who should be considered for RSV vaccination, and reviews the elements of SCDM for RSV vaccines.
The Centers for Disease Control and Prevention (CDC) resource “RSV Vaccination for Adults 60 Years and Older” features brief talking points to consider when engaging in SCDM with patients about RSV vaccination.
At-risk Populations for Severe Infection
RSV causes an annual seasonal respiratory epidemic, which is responsible for as many as 160,000 hospitalizations and 10,000 deaths per year in adults 65 years and older.1 With increased age, individual risk for RSV infection increases, so the ACIP recommends RSV vaccination for adults 60 years and older.
Among this age group, certain patient populations have a further elevated risk for severe RSV-associated disease, including individuals with any of the following chronic conditions2:
- Lung disease (e.g., chronic obstructive pulmonary disease [COPD], asthma)
- Cardiovascular diseases (e.g., congestive heart failure, coronary artery disease)
- Diabetes mellitus
- Neurologic conditions
- Kidney disorders
- Liver disorders
- Hematologic disorders
- Immune compromised
- Other underlying conditions a health care provider determines might increase the risk for severe respiratory disease
In addition to causing lower respiratory illnesses, such as pneumonia, RSV infection can exacerbate underlying conditions, such as asthma, COPD, and congestive heart failure. Patients with these chronic medical conditions should receive special consideration for the RSV vaccine, as they have the greatest risk for severe infection, which can lead to hospital admission and possibly death.
Additional underlying factors may increase the risk of severe RSV-associated respiratory illness for patients 60 years and older, including2 :
- Frailty
- Advanced age
- Residence in a nursing home or other long-term care facility
- Other underlying factors a health care provider determines might increase the risk for severe respiratory disease
Currently Available RSV Vaccines for Patients 60 Years and Older
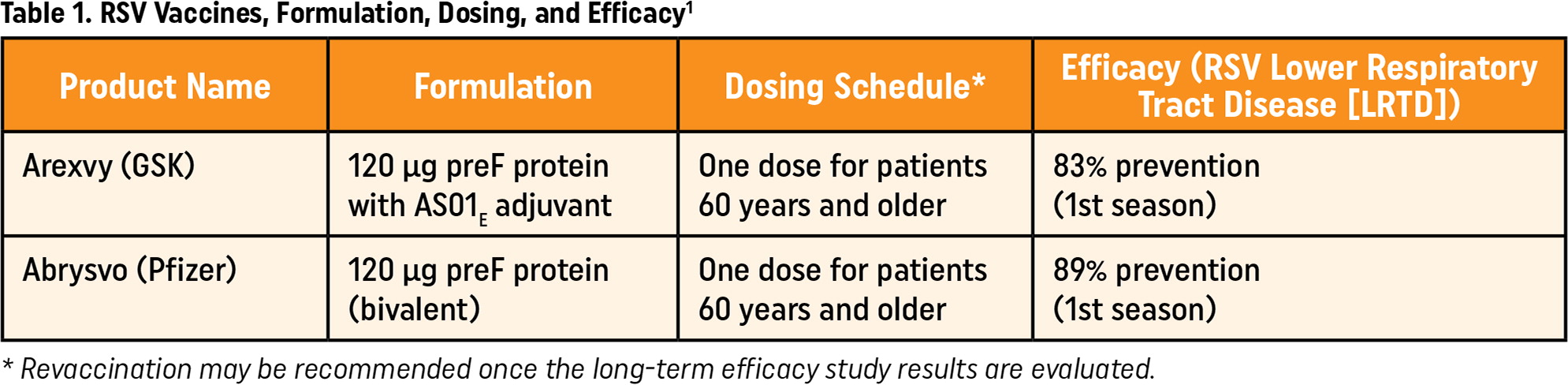
The CDC currently recommends two FDA-approved vaccines for use with SCDM in patients 60 years and older.4,5 Arexvy, manufactured by GSK, is an adjuvant recombinant glycoprotein F vaccine. Abrysvo, produced by Pfizer, is a bivalent recombinant glycoprotein F protein vaccine with efficacy against RSV A and RSV B. Abrysvo is also approved for use in patients who are pregnant from 32 weeks through 36 weeks of gestation from September through January.
Each vaccine is administered as a single dose and comes with a recommendation for SCDM.3 Health care providers should provide recommendations based on patient-specific risk factors that increase the possibility of severe RSV infection or worsening of chronic conditions secondary to RSV infection. Though RSV follows a seasonal pattern with increased risk for infection from October through March, the vaccines for adults 60 years and older can be administered year-round.6
| Product Name | Formulation | Dosing Schedule* | Efficacy (RSV Lower Respiratory Tract Disease [LRTD]) |
|---|---|---|---|
| Arexvy (GSK) | 120 μg preF protein with AS01E adjuvant | One dose for patients 60 years and older | 83% prevention (1st season) |
| Abrysvo (Pfizer) | 120 μg preF protein (bivalent) | One dose for patients 60 years and older | 89% prevention (1st season) |
The current recommendation is to administer the RSV vaccine to appropriate patients as soon as the vaccine is available.1 This differs for RSV vaccination for women who are pregnant and RSV antibody immunization for infants, which are based on seasonal risk for RSV and typically recommended from September to January for women who are pregnant2 and from October to March for infants.7
Vaccine Efficacy
Both RSV vaccines for adults 60 years and older show efficacy in preventing RSV disease for at least two RSV seasons.1 When administered to immune-competent adults 60 years and older, a single dose of RSV vaccine significantly reduces the risk for RSV lower respiratory tract disease (LRTD), such as pneumonia. When considering protection against RSV-related LRTD in the first RSV season following vaccination, Arexvy showed an 83% efficacy, and a single dose of Abrysvo showed an 89% efficacy.9 This protection extends into the second RSV season, with Arexvy remaining 56% effective at protecting against pneumonia in the second RSV season following vaccination. Studies on Abrysvo show additional protection into the second RSV season, but exact efficacy has yet to be determined, with partial second season data showing a 79% efficacy against RSV LRTD.
Vaccine Side Effects and Adverse Events
Common reactions following the Abrysvo vaccination include fatigue, headache, pain at the injection site, and muscle pain.10 Common reactions following the Arexvy vaccination include fatigue, myalgia, headache, pain at the injection site, and arthralgia.11 Both vaccines may carry a low risk of inflammatory neurologic events, such as Guillain-Barré syndrome (GBS).1 Six cases of GBS were reported as serious adverse events during clinical trials — three cases for each vaccine. Atrial fibrillation was also reported as an unsolicited event within 30 days after vaccination for a higher number of participants in the intervention group than in the control group (0.1% for Arexvy; less than 0.1% for Abrysvo). The only contraindication to receiving either RSV vaccine is anaphylaxis to the vaccine or any vaccine component.
Shared Clinical Decision-making
SCDM is intended to add flexibility for physicians and other clinicians advising their patients about vaccination. Unlike universal vaccine recommendations, SCDM “is not a prescribed set of considerations or decision points in the decision-making process.”3 Physicians and other clinicians may find this additional step adds time and complicates medical decision-making, which may create a barrier to administering vaccines to patients who benefit from RSV protection. The key to SCDM is effective communication between physicians and their patients. Knowing this requires added time during a clinical encounter, it may be helpful to plan a brief discussion with patients during their visit and then supplement the clinical discussion with patient-centered resources in an after-visit summary. The patient education article “RSV in Adults Over 60” on the American Academy of Family Physicians' (AAFP) patient website familydoctor.org offers clear language to share with patients about RSV vaccination.
Discussing the role of the RSV vaccine (and vaccines in general) with patients throughout the year and not simply before or during RSV season may allow more time for the patient and physician to determine together whether the patient should receive an RSV vaccine. Physicians should also consider a multi-disciplinary SCDM approach to RSV vaccination. Since Medicare Part D reimburses for RSV vaccination, most patients 65 years and older who receive an RSV vaccine will do so at a pharmacy. The pharmacist is an integral part of the health care team and should be empowered to engage with patients in SCDM for RSV vaccination.3 Appropriate delegation of some of these discussions to the pharmacy team should reduce the time burden for physicians with limited time to address multiple health issues during patient visits.

Barriers to Accessing RSV Immunization
As with any new vaccination, patient awareness is one barrier to administering the vaccine. In 2023, the National Poll on Healthy Aging reported that only 52% of individuals 60–80 years had heard the new RSV vaccine options were coming.12 Addressing this knowledge gap with patients may substantially increase RSV vaccination rates, as this group demonstrated significant interest in receiving RSV vaccination. Sixty percent of individuals in their 60s and 70% in their 70s stated they were very or somewhat interested in receiving the RSV vaccination. Of note, there was a difference in interest when considering race: 65% of white, non-Hispanic individuals were very or somewhat interested, while only 56% of Black and Hispanic individuals were very or somewhat interested in getting an RSV vaccination. Given these differences among various populations, physicians may wish to dedicate additional time and resources to SCDM with these patient groups.
Another potential barrier to receiving an RSV vaccine is knowing where to receive it. Patients with Medicare Part D (the majority of patients 65 years and older) should be fully reimbursed for the RSV vaccine when it is received at a pharmacy.
Individuals with private insurance, such as those 60–64 years or others without Medicare Part D, should confirm with their health insurance provider that the RSV vaccine is covered. Since the RSV vaccine is in the CDC immunization schedule, most insurance providers should cover the cost of the vaccine. However, this may vary based on the insurer.
Finally, one of the main barriers to the RSV vaccine is the time required for SCDM. These discussions may involve multiple interactions throughout several clinical encounters. This discontinuous communication may create inefficiency and magnify the adverse health effects of existing vulnerabilities, such as residing in a medically underserved area, limited health literacy, or lack of transportation — all of which exacerbate health care disparities.13 To reduce vaccination gaps for patients in vulnerable circumstances, care teams should expand the number of care team members engaging in SCDM to include non-clinician team members (e.g., pharmacists, nursing staff, etc.).3 With the expansion, it is important to ensure accurate documentation of prior discussions. This alerts team members of prior vaccine-related discussions, which can enhance the efficiency of subsequent SCDM communication.13 SCDM also creates a unique opportunity to enhance clinical decision support systems. Developing clinical decision support tools could efficiently guide SCDM discussions about RSV vaccines for patients at increased risk.
The CDC offers “Frequently Asked Questions About RSV Vaccine for Adults” as additional clinical guidance.
This AAFP educational supplement is supported by an unrestricted grant from GSK.
