
Am Fam Physician. 2022;106(5):534-542
Author disclosure: No relevant financial relationships.
Adult vaccination rates are low in the United States, despite clear benefits for reducing morbidity and mortality. Vaccine science is evolving rapidly, and family physicians must maintain familiarity with the most recent guidelines. The recommended adult immunization schedule is updated annually by the Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention. All eligible patients should receive SARS-CoV-2 vaccines according to the current guidelines. Adults without contraindications should also receive an annual influenza vaccine. Hepatitis A vaccine is recommended for adults with specific risk factors. All pregnant patients, adults younger than 60 years, and those 60 years and older who have risk factors should receive a hepatitis B vaccine. A 15- or 20-valent pneumococcal conjugate vaccine is recommended for all patients who are 65 years and older. Patients who receive 15-valent pneumococcal conjugate vaccine should receive a dose of 23-valent pneumococcal polysaccharide vaccine one year later. Adults 19 to 64 years of age should receive a pneumococcal vaccination if they have medical risk factors. A single dose of measles, mumps, and rubella vaccine is recommended for adults without presumptive immunity, and additional doses are recommended for patients with HIV and postdelivery for pregnant patients who are not immune to rubella. A tetanus and diphtheria toxoids booster is recommended every 10 years. For pregnant patients and those in close contact with young infants, a tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine should be administered. The human papillomavirus vaccine is recommended for all people through 26 years of age. Herpes zoster vaccine is indicated for all adults 50 years and older. .
Vaccination rates for adults in the United States have not met public health goals; consequently, vaccine-preventable diseases are widespread. Less than optimal vaccination rates may be attributed to variations in perspectives about vaccinations based on cultural and religious beliefs, race, and socioeconomic status.1 Family physicians can positively influence vaccination uptake by educating, reassuring, and recommending vaccination to patients.2 Patient education is important because of the growth of the antivaccination movement.3

| Two doses of a SARS-CoV-2 vaccine decreases the need for hospital care by up to 77% in patients who are immunocompromised and 90% in patients who are immunocompetent. A booster (third) dose provides an additional reduction in severe disease symptoms, and a fourth dose further reduces the short-term risk of disease-related complications. |
| All pregnant patients and adults younger than 60 years should routinely receive hepatitis B vaccination. |
| The Advisory Committee on Immunization Practices recommends recombinant zoster vaccine (Shingrix) for the prevention of herpes zoster and related complications for adults 50 years and older who are immunocompetent. Because of the recombinant zoster vaccine’s greater effectiveness, a full two-dose vaccination series is recommended for adults who previously received the discontinued zoster vaccine live (Zostavax). |
| Primary human papillomavirus vaccination can be administered at as young as nine years of age, before onset of sexual contact, because most new human papillomavirus infections are acquired in adolescence or early adulthood. Catch-up vaccination is now recommended for all people through 26 years of age. |

| Recommendation | Sponsoring organization |
|---|---|
| Do not routinely avoid influenza vaccination in patients who are allergic to eggs. | American Academy of Allergy, Asthma, and Immunology |
Vaccines work by introducing a weakened or inactive bacterial or viral antigen to the body, which starts an immune response through the production of immunoglobulin G and A antibodies. These antibodies bind to the antigen, triggering CD8 T cells to clear infected cells and begin the development of memory CD8 T cells.
The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) vaccination schedule provides updated guidance on vaccine recommendations. Table 1 summarizes the recommendations for vaccines discussed in this article.4 The full CDC vaccination schedule is available at https://www.cdc.gov/vaccines/schedules/hcp/imz/adult.html.
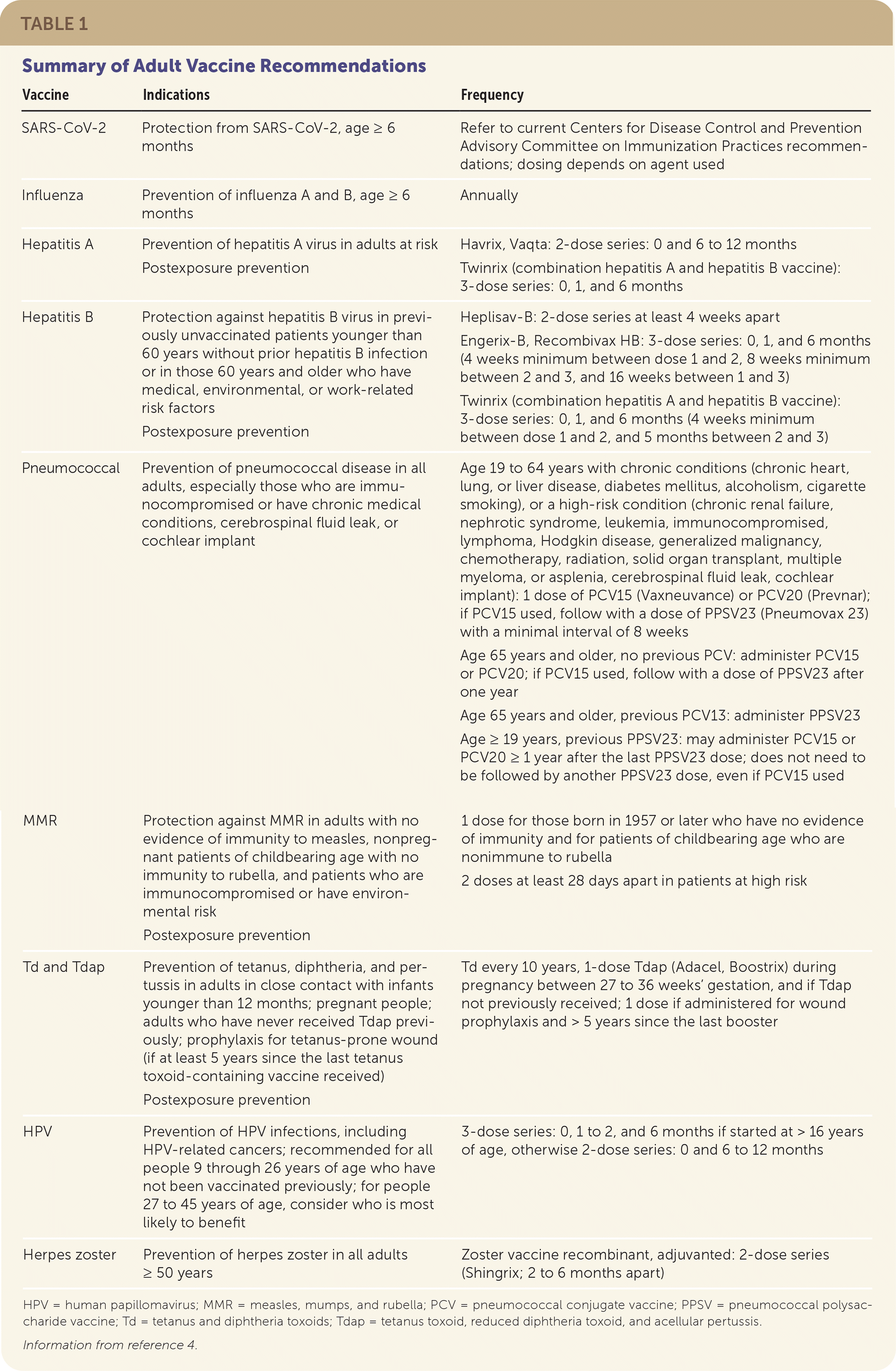
| Vaccine | Indications | Frequency |
|---|---|---|
| SARS-CoV-2 | Protection from SARS-CoV-2, age ≥ 6 months | Refer to current Centers for Disease Control and Prevention Advisory Committee on Immunization Practices recommendations; dosing depends on agent used |
| Influenza | Prevention of influenza A and B, age ≥ 6 months | Annually |
| Hepatitis A | Prevention of hepatitis A virus in adults at risk Postexposure prevention | Havrix, Vaqta: 2-dose series: 0 and 6 to 12 months Twinrix (combination hepatitis A and hepatitis B vaccine): 3-dose series: 0, 1, and 6 months |
| Hepatitis B | Protection against hepatitis B virus in previously unvaccinated patients younger than 60 years without prior hepatitis B infection or in those 60 years and older who have medical, environmental, or work-related risk factors Postexposure prevention | Heplisav-B: 2-dose series at least 4 weeks apart Engerix-B, Recombivax HB: 3-dose series: 0, 1, and 6 months (4 weeks minimum between dose 1 and 2, 8 weeks minimum between 2 and 3, and 16 weeks between 1 and 3) Twinrix (combination hepatitis A and hepatitis B vaccine): 3-dose series: 0, 1, and 6 months (4 weeks minimum between dose 1 and 2, and 5 months between 2 and 3) |
| Pneumococcal | Prevention of pneumococcal disease in all adults, especially those who are immunocompromised or have chronic medical conditions, cerebrospinal fluid leak, or cochlear implant | Age 19 to 64 years with chronic conditions (chronic heart, lung, or liver disease, diabetes mellitus, alcoholism, cigarette smoking), or a high-risk condition (chronic renal failure, nephrotic syndrome, leukemia, immunocompromised, lymphoma, Hodgkin disease, generalized malignancy, chemotherapy, radiation, solid organ transplant, multiple myeloma, or asplenia, cerebrospinal fluid leak, cochlear implant): 1 dose of PCV15 (Vaxneuvance) or PCV20 (Prevnar); if PCV15 used, follow with a dose of PPSV23 (Pneumovax 23) with a minimal interval of 8 weeks Age 65 years and older, no previous PCV: administer PCV15 or PCV20; if PCV15 used, follow with a dose of PPSV23 after one year Age 65 years and older, previous PCV13: administer PPSV23 Age ≥ 19 years, previous PPSV23: may administer PCV15 or PCV20 ≥ 1 year after the last PPSV23 dose; does not need to be followed by another PPSV23 dose, even if PCV15 used |
| MMR | Protection against MMR in adults with no evidence of immunity to measles, nonpregnant patients of childbearing age with no immunity to rubella, and patients who are immunocompromised or have environmental risk Postexposure prevention | 1 dose for those born in 1957 or later who have no evidence of immunity and for patients of childbearing age who are nonimmune to rubella 2 doses at least 28 days apart in patients at high risk |
| Td and Tdap | Prevention of tetanus, diphtheria, and pertussis in adults in close contact with infants younger than 12 months; pregnant people; adults who have never received Tdap previously; prophylaxis for tetanus-prone wound (if at least 5 years since the last tetanus toxoid-containing vaccine received) Postexposure prevention | Td every 10 years, 1-dose Tdap (Adacel, Boostrix) during pregnancy between 27 to 36 weeks’ gestation, and if Tdap not previously received; 1 dose if administered for wound prophylaxis and > 5 years since the last booster |
| HPV | Prevention of HPV infections, including HPV-related cancers; recommended for all people 9 through 26 years of age who have not been vaccinated previously; for people 27 to 45 years of age, consider who is most likely to benefit | 3-dose series: 0, 1 to 2, and 6 months if started at > 16 years of age, otherwise 2-dose series: 0 and 6 to 12 months |
| Herpes zoster | Prevention of herpes zoster in all adults ≥ 50 years | Zoster vaccine recombinant, adjuvanted: 2-dose series (Shingrix; 2 to 6 months apart) |
Insurance coverage for vaccines differs by payor. Beginning in January 2023, all Medicare Part D plans will be required to cover adult vaccines recommended by ACIP with no cost sharing, even if the beneficiary is in the deductible phase of benefits. Medicaid will implement a similar requirement beginning in October 2023.
SARS-CoV-2 Vaccine (COVID-19 Vaccine)
Three SARS-CoV-2 vaccines have been developed and approved in the United States. The Pfizer and Moderna vaccines contain a single-stranded messenger RNA (mRNA) molecule, which is transcribed by white blood T cells into a protein antigen that triggers an immune response.5 This type of SARS-CoV-2 vaccine was developed based on mRNA technology used in vaccines for Zika virus infections, HIV-1, influenza, and solid organ tumors.6 The Johnson & Johnson (Janssen) COVID-19 vaccine is a viral vector vaccine. Due to the risk of adverse events, including thrombosis and thrombocytopenia, the Johnson & Johnson (Janssen) COVID-19 vaccine is no longer preferred.7
The Pfizer and Moderna vaccines have shown high effectiveness. Clinicians should administer an mRNA SARSCoV-2 vaccine to eligible patients to decrease the risk of hospitalization and death.8,9 Two doses of a COVID-19 vaccine decreases the need for hospital care by up to 77% in patients who are immunocompromised and up to 90% in patients who are immunocompetent.10
As a result of the emergence of the Omicron strain, the U.S. Food and Drug Administration (FDA) no longer authorizes booster doses of the original monovalent Pfizer and Moderna vaccines for adults. New bivalent Pfizer and Moderna booster vaccines have received emergency use authorization from the FDA. As of September 8, 2022, the CDC recommends that a single booster dose of either of the new bivalent COVID-19 vaccines be administered to adults at least two months after completing a primary two-dose vaccine series with either of the original monovalent vaccines or having received a booster dose with one of the monovalent vaccines.11
As a result of the evolution in the timing and frequency of additional boosters and potential changes in antigen components when different COVID-19 variants emerge, clinicians should monitor updates on the CDC and the FDA websites about these and other vaccines (Table 2).
| Topic | Website | Description |
|---|---|---|
| Adult vaccine schedule | https://www.cdc.gov/vaccines/schedules/hcp/imz/adult.html | Recommendations for vaccine use in the United States, updated annually |
| Advisory Committee on Immunization Practices vaccine recommendations and guidelines | https://www.cdc.gov/vaccines/hcp/acip-recs/index.html | Detailed vaccine guidelines and references |
| Vaccine for travelers | https://wwwnc.cdc.gov/travel | Updated regularly based on disease surveillance and risk |
| Vaccine news and updates | https://www.cdc.gov/vaccines/news/related.html | Reliable source of new information and updates |
| Adverse events | https://vaers.hhs.gov | Where to report vaccine-associated adverse events |
| U.S. Food and Drug Administration | https://www.fda.gov/vaccines-blood-biologics/vaccines | Licensing and safety information for vaccines in the United States |
Influenza Vaccines
Influenza vaccines can be classified as inactivated, live attenuated, or recombinant (Table 3).12 The latter is produced with an influenza DNA sequence inserted into a baculovirus (rather than using chicken eggs).12 That virus then produces the antigen for the vaccine. All influenza vaccines are quadrivalent vaccines designed to protect against two influenza A and two influenza B viruses.13
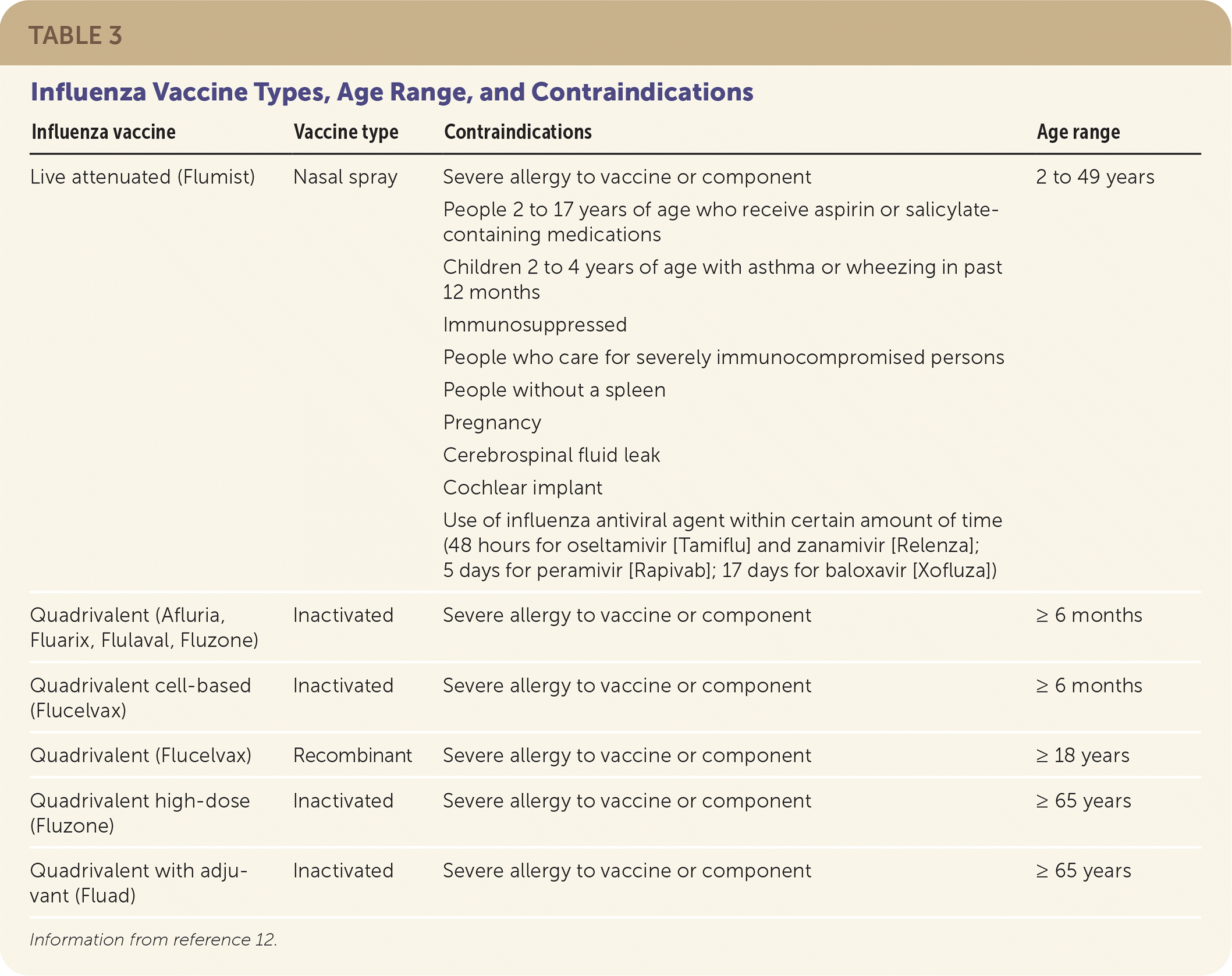
| Influenza vaccine | Vaccine type | Contraindications | Age range |
|---|---|---|---|
| Live attenuated (Flumist) | Nasal spray | Severe allergy to vaccine or component People 2 to 17 years of age who receive aspirin or salicylate-containing medications Children 2 to 4 years of age with asthma or wheezing in past 12 months Immunosuppressed People who care for severely immunocompromised persons People without a spleen Pregnancy Cerebrospinal fluid leak Cochlear implant Use of influenza antiviral agent within certain amount of time (48 hours for oseltamivir [Tamiflu] and zanamivir [Relenza]; 5 days for peramivir [Rapivab]; 17 days for baloxavir [Xofluza]) | 2 to 49 years |
| Quadrivalent (Afluria, Fluarix, Flulaval, Fluzone) | Inactivated | Severe allergy to vaccine or component | ≥ 6 months |
| Quadrivalent cell-based (Flucelvax) | Inactivated | Severe allergy to vaccine or component | ≥ 6 months |
| Quadrivalent (Flucelvax) | Recombinant | Severe allergy to vaccine or component | ≥ 18 years |
| Quadrivalent high-dose (Fluzone) | Inactivated | Severe allergy to vaccine or component | ≥ 65 years |
| Quadrivalent with adjuvant (Fluad) | Inactivated | Severe allergy to vaccine or component | ≥ 65 years |
The CDC provides guidance about which type of vaccine should be used based on age and contraindications. Therefore, one vaccine may be preferred over others for individual patients.14 People with an egg allergy of any severity can receive any licensed, age-appropriate influenza vaccine. People with severe reactions can be vaccinated under the supervision of a health care professional.15
The ACIP recommends that all patients six months and older who do not have contraindications receive routine annual influenza vaccination.4 Systematic reviews have found that influenza vaccines can reduce the proportion of adults, including older adults, who develop influenza illness by about one-half (2.3% risk in healthy unvaccinated adults vs. 1% in vaccinated adults).16,17
Hepatitis A Vaccine
Hepatitis A vaccination (Havrix, Vaqta) is recommended for adults when specific risk factors are present (Table 4).18 Hepatitis A vaccination should also be administered to adults within two weeks of a known exposure.19 A combination vaccine with hepatitis B (Twinrix) can be administered when both vaccines are indicated for travelers to areas with endemic diseases, patients with hepatitis C or chronic liver disease, and other individuals who are at high risk.

| Increased risk of hepatitis A |
| Close personal contact (e.g., household, caretaker, sexual) with people who have hepatitis A |
| International travelers |
| Men who have sex with men |
| People experiencing homelessness |
| People who anticipate close personal contact with an international adoptee |
| People who use injection or noninjection drugs (i.e., all those who use illegal drugs) |
| People with occupational risk of exposure |
| Increased risk of severe disease from hepatitis A virus infection |
| People > 40 years |
| People with chronic liver disease* |
| People with HIV infection |
| Other |
| Pregnant people at risk of hepatitis A or severe outcome from hepatitis A |
| Any person who requests vaccination |
| Vaccination during outbreaks |
| Unvaccinated people in outbreak settings who are at risk of hepatitis A or severe disease from hepatitis A |
| Implementation strategies for settings providing services to adults† |
| People in settings that provide services to adults in which a high proportion of those persons have risk factors for hepatitis A |
| Hepatitis A vaccination is no longer recommended by the Advisory Committee on Immunization Practices |
| People who receive blood products for clotting disorders (e.g., hemophilia) |
Hepatitis B Vaccine
Hepatitis B vaccines are recombinant single- or multiple-antigen vaccines. The multiple-antigen vaccines (Engerix-B, Recombivax HB, Twinrix) can be administered as a three-dose series at zero, one, and six months. The HepB-CpG vaccine (Heplisav-B) is a single-antigen vaccine using a novel immune-stimulatory sequence adjuvant that can be administered in two doses, four weeks apart.20
All pregnant patients and adults younger than 60 years should be vaccinated for hepatitis B. Although hepatitis B vaccines can safely be administered to patients 60 years and older, it is specifically recommended for those in that age group who are at risk of infection (i.e., chronic liver disease, HIV infection, sexual exposure risk, injection drug use, risk of exposure, incarceration, or travel to areas with a high or intermediate endemic virus). Hepatitis B vaccines should not be administered to those who have had an allergic reaction to yeast or neomycin.21
Pneumococcal Vaccines
Four pneumococcal vaccines are licensed in the United States for adults: 13-valent pneumococcal conjugate vaccine (PCV13; Prevnar 13), 15-valent pneumococcal conjugate vaccine (PCV15; Vaxneuvance), 20-valent pneumococcal conjugate vaccine (PCV20; Prevnar 20), and a 23-valent pneumococcal polysaccharide vaccine (PPSV23; Pneumovax 23).22 PCV13 is no longer part of the current immunization schedule for adults.
It is recommended that adults 65 years and older who have not previously received a PCV should receive one dose of PCV15 or PCV20. If PCV15 is administered, it should be followed by a dose of PPSV23 after one year; however, the interval can be shortened to eight weeks for individuals with immunocompromising conditions, cerebral spinal fluid leak, or cochlear implant.22,23 If PCV20 is used for the first pneumococcal immunization, a subsequent dose of PPSV23 is not indicated.
Vaccination with one dose of PCV15 or PCV20 is recommended for adults 19 to 64 years of age with medical risk factors, including chronic heart, lung, or liver disease; diabetes mellitus; alcoholism; or cigarette smoking; and patients with immunocompromising conditions. If PCV15 is used, it should be followed by a dose of PPSV23. If PCV20 is used, subsequent administration of PPSV23 is not indicated. In adults with risk factors who have received PPSV23 alone, PCV20 or PCV15 may be administered after at least one year.24
Measles, Mumps, and Rubella Vaccine
The live, attenuated measles, mumps, and rubella (MMR) vaccine is recommended for adults without presumptive or confirmed evidence of immunity. Such evidence includes documentation of receiving one or more doses of a measles-containing vaccine for adults not at high risk or two doses for adults at high risk (e.g., health care workers, college students, international travelers), laboratory evidence of immunity, or birth before 1957.
One dose of the MMR vaccine is recommended for non-pregnant patients of childbearing age without presumptive or confirmed immunity to rubella. Two doses are recommended for patients with HIV.4 Among those who have completed the two-dose MMR series, a third dose of the vaccine decreases the risk of subsequent infection during an outbreak.25 If a patient is found to be rubella nonimmune during pregnancy, a booster dose of MMR is recommended postdelivery.
Td and Tdap Vaccines
A tetanus and diphtheria toxoids (Td) vaccine or tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine is recommended every 10 years. However, the Tdap vaccine is indicated for adults in close contact with infants younger than 12 months; during pregnancy between 27 and 36 weeks’ gestation regardless of previous boosters, with an emphasis on administration in the earlier part of this range; and for individuals who have not previously received a Tdap vaccine. Administration during pregnancy may reduce neonatal tetanus, and decreases the risk of pertussis in neonates until they are old enough to receive their first pertussis vaccine at two months.26
Postwound tetanus prophylaxis is recommended with Td or Tdap if it has been at least five years since the last tetanus vaccine. There are two types of FDA-approved Tdap vaccines. Adacel and Boostrix are made with tetanus and diphtheria toxoids and inactivated pertussis antigen. Adacel, however, is only FDA-approved for patients between 10 and 64 years of age. Boostrix can be administered to patients 10 years and older who require the Tdap vaccine.4
Human Papillomavirus Vaccine
Human papillomavirus (HPV) vaccination is highly effective in preventing HPV-associated cancer. The primary benefit for women is a reduction of cervical cancer.27 The benefit of HPV vaccination in men includes a reduction of external genital lesions with the potential to reduce anal intraepithelial neoplasia and head or neck cancers.28,29
Primary HPV vaccination can be administered at as young as nine years of age, before the onset of sexual contact, because most new HPV infections are acquired in adolescence or early adulthood. However, catch-up vaccination is now recommended through 26 years of age.29 Vaccination is not recommended during pregnancy.29
Adults at any age who have a new sex partner are also at risk of HPV exposure. Shared clinical decision-making to determine the benefit of vaccination is recommended for adults 27 to 45 years of age.29
HPV vaccination is FDA-approved for patients between nine and 45 years of age. Women between 21 and 64 years of age, regardless of their HPV vaccination status, should receive cervical cancer screening because the vaccine does not protect against all subtypes of HPV. There is no HPV screening test for men.
Varicella Vaccine
Adults who have not had varicella (chickenpox) or the vaccine should receive two doses of varicella vaccine at least 28 days apart.4 Adults born before 1980, except for pregnant patients and health care personnel, are considered immune. Varicella vaccine is indicated in the post-partum period for patients who were not immune during pregnancy.4
Herpes Zoster Vaccine
Herpes zoster vaccine is a nonlive, recombinant, adjuvanted, glycoprotein vaccine known as a recombinant zoster vaccine (Shingrix). The herpes zoster vaccine is a two-dose vaccine administered intramuscularly, two to six months apart, and is the only zoster vaccine available in the United States. The zoster vaccine live (Zostavax) is no longer available.
The herpes zoster vaccine decreases the risk of shingles and postherpetic neuralgia by more than 90% in people 50 years and older.30 The ACIP recommends the herpes zoster vaccine for the prevention of herpes zoster and related complications for adults 50 years and older who are immunocompetent. Because of the herpes zoster vaccine’s greater effectiveness, a two-dose vaccination series is recommended for adults who previously received the discontinued zoster vaccine live.
The herpes zoster vaccine is also indicated for people 19 years and older who are immunocompromised, including those on immunosuppressive therapy (optimally administered before starting that therapy) or recovering from an immunocompromising illness.31
The ACIP recommends that people with a history of herpes zoster be vaccinated because the disease can recur. It is not necessary to screen for a history of varicella or test for a previous infection before administering the herpes zoster vaccine.30
Monkeypox Vaccine
As of September 2022, there were nearly 25,000 cases of monkeypox in the United States.32 This has led the CDC to issue recommendations for the general use of the two vaccines that had been previously recommended by the ACIP for preventing monkeypox in people with occupational exposure, with the goal of preventing monkeypox in individuals at personal risk of exposure (i.e., nonoccupational risk).33
The CDC recommends vaccination for individuals whose sex partner has been diagnosed with monkeypox within the past two weeks, individuals who have had close contact with someone who has monkeypox, and men who have sex with men or have had multiple sex partners within the past two weeks in an area where there has been monkeypox transmission.33
There are two monkeypox vaccines available in the United States. The JYNNEOS vaccine was developed to protect against smallpox and monkeypox. The ACAM2000 vaccine was developed to protect against smallpox but is also expected to provide protection against monkeypox.33 The JYNNEOS vaccine is mainly being used during the current outbreak, with ACAM2000 considered only as an alternative because it has more adverse effects and is not recommended for people who are immunocompromised.33 The JYNNEOS vaccine is a two-dose series, administered 28 days apart. COVID-19 vaccines should be delayed by four weeks in patients receiving the JYNNEOS vaccine.34
Monkeypox is an evolving topic with little data to support clinical effectiveness of these vaccines in the current outbreak. The CDC website should be monitored for updates (https://www.cdc.gov/poxvirus/monkeypox/health-departments/vaccine-considerations.html).
Other Vaccines
The CDC provides guidance on vaccines for individuals with specific exposure risks or those traveling to areas with endemic or epidemic vaccine-preventable illnesses. Available vaccines include those for meningitis, typhoid, cholera, yellow fever, Japanese encephalitis, rabies, and malaria. For travel to areas with an increased risk of polio, adults who have completed the polio vaccine series during childhood should receive a one-time booster dose of inactivated polio vaccine. The ACIP vaccine schedule and the traveler’s health section of the CDC website have specific guidance for these vaccines based on individual risk.
This article updates a previous article on this topic by Vaughn and Miller.35
Data Sources: A search in PubMed and Essential Evidence Plus was conducted using the key terms immunization, adult immunization, vaccine, and covid. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. Also searched was the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices. Search dates: June 30, 2021; December 7, 2021; May 7, 2022; and August 31, 2022.
Dr. Hanson passed away while this article was being developed. She was an outstanding leader, mentor, and role model. Her legacy lives on in the many students and patients whom she positively influenced over the course of her career. She will be greatly missed.
