
Am Fam Physician. 2022;106(5):513-522
Related Letter: Harm Reduction for Patients Who Smoke
Related Letter: Integrated Policy Actions for Smoking Cessation
Related Letter: Smoking Cessation in Adolescents
Patient information: See related handout on smoking cessation.
Author disclosure: No relevant financial relationships.
In the United States, 1 in 5 adults uses tobacco products. Cigarette smoking is the leading cause of preventable disease and death in the United States despite its known health effects. Although nearly one-half of people who smoke try to quit each year, only up to 1 in 20 who quit without support achieve abstinence for at least six months. All patients, including school-aged children and adolescents, should be asked if they smoke and offered evidence-based treatments for smoking cessation. Use of the 5 A’s framework (ask, advise, assess, assist, arrange) can help clinicians promote smoking cessation. Clinical studies have demonstrated that combining pharmacotherapy with effective behavior strategies is significantly more effective than either approach alone. Pharmacotherapies approved by the U.S. Food and Drug Administration for smoking cessation include nicotine replacement therapy, bupropion, and varenicline. Extended use (greater than 12 weeks) of a controller therapy (varenicline, bupropion, or nicotine patch) is associated with significantly higher sustained quit rates and lower relapse rates than standard use (six to 12 weeks). e-Cigarettes are not approved by the U.S. Food and Drug Administration for smoking cessation, and evidence supporting their benefit is inconclusive. Lung cancer screening is recommended for adults 50 to 80 years of age who have a 20-pack-year smoking history and currently smoke or have quit within the past 15 years. Lung cancer screening should be combined with smoking cessation tools and treatment.
In the United States, 1 in 5 adults uses tobacco products. Cigarettes and other forms of combustible tobacco products (cigars and pipes) account for 80.5% of tobacco use.1 Despite the known health effects of cigarettes, smoking is the leading cause of preventable disease and death in the United States.1
| Clinical recommendation | Evidence rating | Comments |
|---|---|---|
| All patients should be asked about tobacco use and advised to quit.5,6 | A | U.S. Public Health Service report and USPSTF guideline |
| All nonpregnant adults who smoke cigarettes should be offered pharmacotherapy and behavior interventions to help cessation attempts.6 | A | USPSTF guideline and a systematic overview of 64 systematic reviews |
| Behavior interventions combined with pharmacotherapy improve smoking cessation rates.6,12,13 | A | Systematic reviews demonstrating that combined interventions are more effective than usual care or minimal intervention |
| Prescribe varenicline to patients willing to take varenicline even if they are not ready to quit smoking.9 | B | Systematic review of four RCTs showing that varenicline increases abstinence at six months, even in patients reluctant to quit, with few adverse effects; American Thoracic Society guideline reporting high certainty of benefits |
| All pregnant people should be screened for tobacco use, advised to quit, and provided behavior interventions to help with cessation.6,47 | A | USPSTF guideline with seven RCTs and five large observational studies; the benefits and risks of nicotine replacement therapy in pregnant people should be balanced against the risk of continued tobacco use |
| School-aged children and adolescents younger than 18 years should be asked about tobacco and e-cigarette use and provided with interventions to prevent initiation of tobacco use. There is insufficient evidence to recommend behavior interventions or pharmacotherapy to help cessation efforts in this population.51 | B | Strategies to prevent initiation of tobacco use include counseling and education; in school-aged children and adolescents, most smoking cessation studies were heterogenous and underpowered to determine the effectiveness of cessation interventions |
Almost one-half of people who smoke try to quit each year; however, only up to 1 in 20 of those who quit without support achieve abstinence for at least six months. Brief discussions with clinicians can increase cessation rates by two-thirds, and more intensive treatment doubles the chances of quitting.2
Recommending Smoking Cessation
Including smoking status with the vital sign assessment has not resulted in increased smoking cessation rates. Despite doubling the documentation of patient smoking status from 38% to 78%, clinicians are not addressing smoking more often.3 Studies have shown that clinicians who are trained in smoking cessation and knowledgeable about local resources are more likely to provide smoking cessation interventions.4 Therefore, clinician training and engagement are important variables in smoking cessation interventions.
The American Academy of Family Physician’s (AAFP’s) Ask and Act Tobacco Cessation Program provides free resources and tools to aid physicians (https://www.aafp.org/family-physician/patient-care/care-resources/tobacco-and-nicotine/ask-act.html). All patients should be asked if they smoke, regardless of age, sex, or medical history. This includes school-aged children and adolescents because more than one-half of adult smokers start smoking daily before 18 years of age.5,6
The U.S. Public Health Service suggests using the 5 A’s framework (ask each patient about tobacco use, advise cessation, assess readiness to quit, assist tobacco users in quitting, arrange follow-up visits) in the outpatient setting5 (Table 15–9). Acknowledging that clinicians may not have adequate time and may not feel comfortable treating tobacco use disorders, simplified models such as AAR (ask, advise, refer to treatment resources) or AAC (ask, advise, connect patients to a quitline via fax, phone, or electronic referral) can be used.4
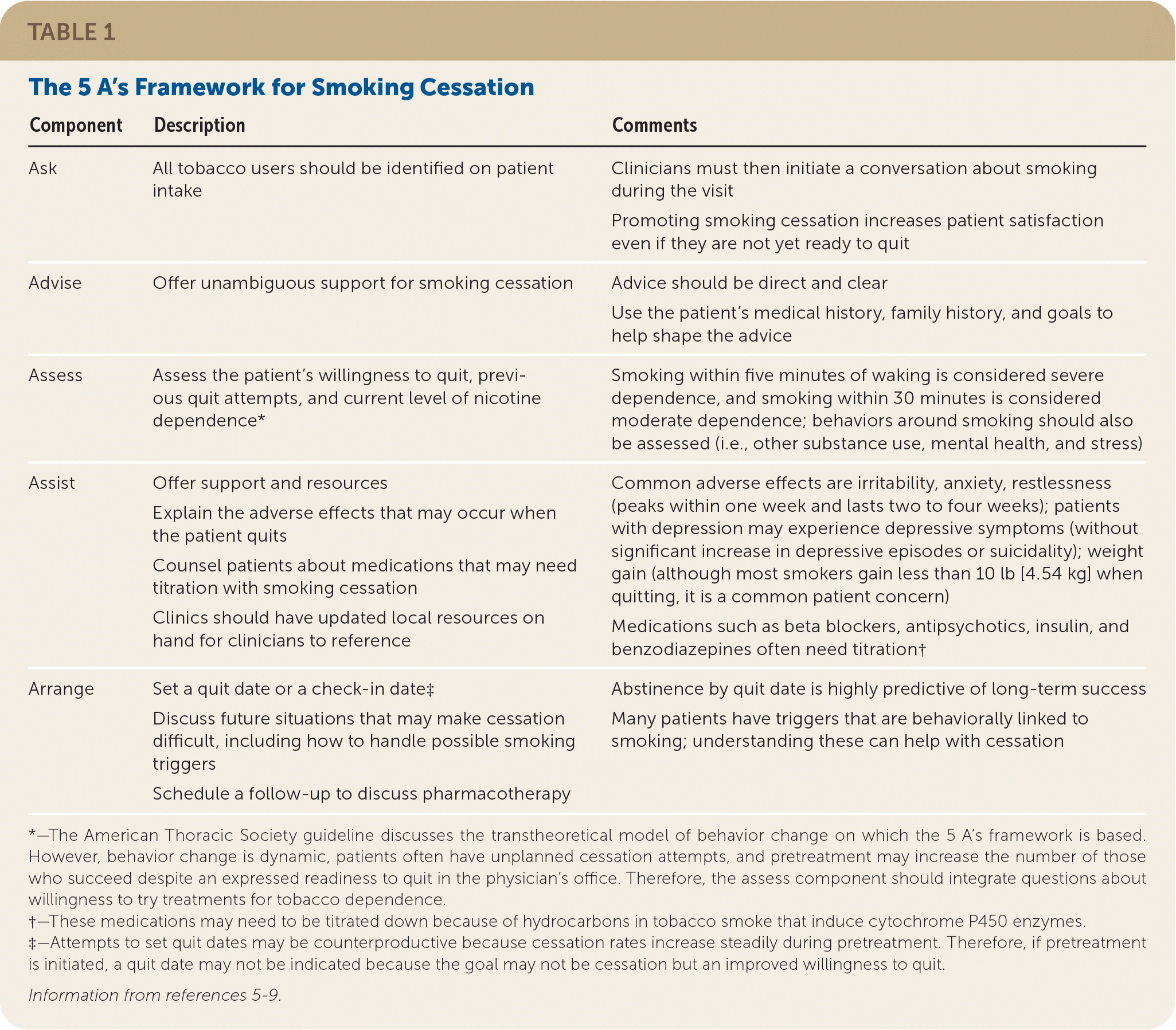
| Component | Description | Comments |
|---|---|---|
| Ask | All tobacco users should be identified on patient intake | Clinicians must then initiate a conversation about smoking during the visit Promoting smoking cessation increases patient satisfaction even if they are not yet ready to quit |
| Advise | Offer unambiguous support for smoking cessation | Advice should be direct and clear Use the patient’s medical history, family history, and goals to help shape the advice |
| Assess | Assess the patient’s willingness to quit, previous quit attempts, and current level of nicotine dependence* | Smoking within five minutes of waking is considered severe dependence, and smoking within 30 minutes is considered moderate dependence; behaviors around smoking should also be assessed (i.e., other substance use, mental health, and stress) |
| Assist | Offer support and resources Explain the adverse effects that may occur when the patient quits Counsel patients about medications that may need titration with smoking cessation Clinics should have updated local resources on hand for clinicians to reference | Common adverse effects are irritability, anxiety, restlessness (peaks within one week and lasts two to four weeks); patients with depression may experience depressive symptoms (without significant increase in depressive episodes or suicidality); weight gain (although most smokers gain less than 10 lb [4.54 kg] when quitting, it is a common patient concern) Medications such as beta blockers, antipsychotics, insulin, and benzodiazepines often need titration† |
| Arrange | Set a quit date or a check-in date‡ Discuss future situations that may make cessation difficult, including how to handle possible smoking triggers Schedule a follow-up to discuss pharmacotherapy | Abstinence by quit date is highly predictive of long-term success Many patients have triggers that are behaviorally linked to smoking; understanding these can help with cessation |
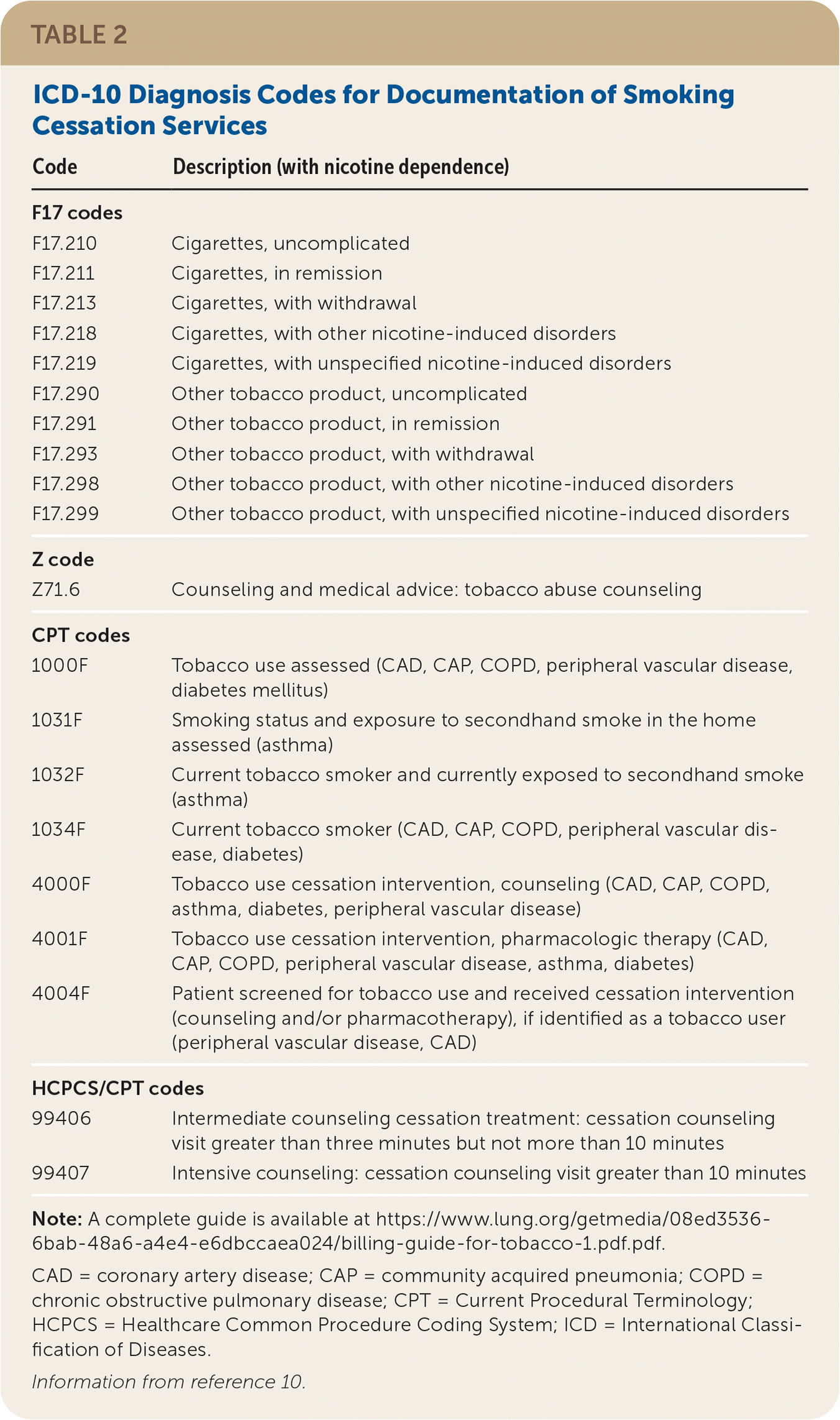
| Code | Description (with nicotine dependence) |
|---|---|
| F17 codes | |
| F17.210 | Cigarettes, uncomplicated |
| F17.211 | Cigarettes, in remission |
| F17.213 | Cigarettes, with withdrawal |
| F17.218 | Cigarettes, with other nicotine-induced disorders |
| F17.219 | Cigarettes, with unspecified nicotine-induced disorders |
| F17.290 | Other tobacco product, uncomplicated |
| F17.291 | Other tobacco product, in remission |
| F17.293 | Other tobacco product, with withdrawal |
| F17.298 | Other tobacco product, with other nicotine-induced disorders |
| F17.299 | Other tobacco product, with unspecified nicotine-induced disorders |
| Z code | |
| Z71.6 | Counseling and medical advice: tobacco abuse counseling |
| CPT codes | |
| 1000F | Tobacco use assessed (CAD, CAP, COPD, peripheral vascular disease, diabetes mellitus) |
| 1031F | Smoking status and exposure to secondhand smoke in the home assessed (asthma) |
| 1032F | Current tobacco smoker and currently exposed to secondhand smoke (asthma) |
| 1034F | Current tobacco smoker (CAD, CAP, COPD, peripheral vascular disease, diabetes) |
| 4000F | Tobacco use cessation intervention, counseling (CAD, CAP, COPD, asthma, diabetes, peripheral vascular disease) |
| 4001F | Tobacco use cessation intervention, pharmacologic therapy (CAD, CAP, COPD, peripheral vascular disease, asthma, diabetes) |
| 4004F | Patient screened for tobacco use and received cessation intervention (counseling and/or pharmacotherapy), if identified as a tobacco user (peripheral vascular disease, CAD) |
| HCPCS/CPT codes | |
| 99406 | Intermediate counseling cessation treatment: cessation counseling visit greater than three minutes but not more than 10 minutes |
| 99407 | Intensive counseling: cessation counseling visit greater than 10 minutes |
Pharmacotherapy
All nonpregnant adults who smoke cigarettes should be offered pharmacotherapy for smoking cessation, especially patients who meet the criteria for nicotine dependence.2 The U.S. Preventive Services Task Force (USPSTF) recommends pharmacotherapy, alone or combined with behavior strategies, for smoking cessation, and the AAFP supports this recommendation.6,11 Clinical studies have demonstrated that combining pharmacotherapy with behavior strategies significantly improves quit success compared with usual care or minimal intervention.6,12 Adding behavior support to pharmacotherapy is more effective than pharmacotherapy alone.6,13
There are seven pharmacotherapies approved by the U.S. Food and Drug Administration (FDA) for smoking cessation: nicotine replacement therapy (NRT) in the form of a transdermal patch, gum, lozenge, inhaler, and nasal spray; bupropion; and varenicline (Chantix). These therapies are identified as controllers or relievers based on their duration of action. Controllers (i.e., bupropion, varenicline, and nicotine patch) help reduce the impulse to smoke, whereas relievers (short-acting NRTs) aid in managing acute cravings. Table 3 summarizes FDA-approved therapies for smoking cessation.8,14,15
| Therapy | Formulations/maximum dose | Instructions for use | Adverse effects | Comments |
|---|---|---|---|---|
| Controllers | ||||
| Bupropion | 150-mg extended-release tablet Maximum = 300 mg per day | 150 mg per day for three days, then 150 mg twice per day with at least eight hours between doses | Insomnia, anxiety, difficulty concentrating, dry mouth, headache, rash, nausea, dizziness, constipation, seizures (1 per 1,000 patients) | Prescription May delay weight gain associated with quitting; may be helpful for patients with coexisting depression Contraindicated in patients with a history of seizures or eating disorders, who are taking medications that lower seizure threshold, or who have used an MAOI in the preceding 14 days Also contraindicated with abrupt cessation of alcohol, barbitu-rates, benzodiazepines, or antiepileptics Dose adjustment recommended for hepatic impairment |
| Varenicline (Chantix) | 0.5- or 1-mg tablet | Start one week before quit date; alternatively, start while smoking and quit on day 8 to 35 of treatment Take 0.5 mg once per day for three days, then 0.5 mg twice per day for four days, then 1 mg twice per day until the end of treatment (12 weeks); take with food and a full glass of water If patients need to decrease their smoking gradually, consider reducing smoking by 50% by week 4 of treatment, by another 50% by week 8, and then reach abstinence by week 12 Continue treatment for an additional 12 weeks after cessation | Nausea, vomiting, constipation, flatulence, insomnia, abnormal dreams, headache, fatigue | Prescription Dose adjustment is recommended for stage 4 kidney disease No difference in the risk of neuropsychiatric events for patients with and without psychiatric illness compared with placebo; U.S. Food and Drug Administration boxed warning has been removed |
| Nicotine transdermal patch* | 7-, 14-, or 21-mg extended-release patch | Patches should be changed daily For heavy smokers (> 10 cigarettes per day), start at 21 mg per day and gradually taper; suggested regimen is 21 mg for four weeks, then 14 mg for two weeks, then 7 mg for two weeks For smokers of ≤ 10 cigarettes per day, start at 14 mg per day and gradually taper; suggested regimen is 14 mg for six weeks, then 7 mg for two weeks | Skin reactions (erythema, itching, burning), headache, sleep disturbances (insomnia, vivid dreams) | Prescription and OTC May remove patch at bedtime if associated with significant sleep disturbances; not advised for use in patients with severe eczema, psoriasis, or other major skin disorder; should be removed before magnetic resonance imaging to prevent burns |
| Relievers* | ||||
| Nicotine gum | 2- or 4-mg gum Maximum = 24 pieces per day | Preload or start on quit date Chew gum slowly, and park between cheek and gums once peppery taste or tingling sensation occurs; resume chewing when taste or tingling fades Repeat chew and park steps Discard gum when taste or tingling does not return with chewing Avoid food and drink for 15 minutes before and after use Use 4-mg dose if craving cigarettes within 30 minutes of waking, otherwise use 2-mg dose Titrate according to withdrawal symptoms and cravings Suggested regimen is one piece every one to two hours for weeks 1 to 6, one piece every two to four hours for weeks 7 to 9, one piece every four to eight hours for weeks 10 to 12; treatment duration is 12 weeks | Jaw pain, hiccups, dyspepsia, hypersalivation, lightheadedness, nausea, vomiting; throat or mouth irritation can be mitigated by reinforcing proper chewing technique | Prescription or OTC May delay weight gain associated with quitting Use with caution in patients with temporomandibular joint arthritis, dentures, or other significant dental work |
| Nicotine lozenge | 2- or 4-mg lozenge (standard size and mini) Maximum = 20 lozenges per day, or five lozenges in six hours | Preload or start on quit date Park between cheek and gums and allow to dissolve slowly (20 to 30 minutes for standard, 10 minutes for mini) Should not be chewed or swallowed; instructions for use are otherwise identical to nicotine gum; treatment duration is 12 weeks | Nausea, hiccups, headache, cough, heartburn, flatulence, insomnia | OTC May delay weight gain associated with quitting |
| Nicotine nasal spray | 10 mg per mL metered spray One dose = one spray in each nostril; one spray delivers 0.5 mg of nicotine Maximum = five doses per hour, or 40 doses per day | Preload or start on quit date Spray once into each nostril (do not sniff, swallow, or inhale while spraying) Wait two to three minutes before blowing nose Use one to two doses per hour and titrate according to withdrawal symptoms and cravings Treatment duration is up to six months | Burning sensation in nose and throat, rhinitis, watery eyes, cough, sneezing, headache | Prescription only Should be avoided in patients with chronic sinusitis, nasal polyps, rhinitis, and severe reactive airway disease |
| Nicotine oral inhaler | 10-mg cartridge; one cartridge delivers 4 mg of inhaled vapor Maximum = 16 cartridges per day | Preload or start on quit date Take short, shallow puffs into the back of the throat (do not inhale into the lungs like an asthma inhaler or cigarette); continue for 20 minutes Avoid food and drink for 15 minutes before and after use Use one cartridge every one to two hours and titrate according to withdrawal symptoms and cravings Treatment duration is up to 12 weeks, followed by gradual taper for six to 12 weeks | Mouth and throat irritation, cough, headache, rhinitis, dyspepsia, hiccups | Prescription Mimics hand-to-mouth action of smoking Less effective at temperatures < 60°F (15.5°C) Should be avoided in patients with bronchospasms |
Traditionally, treatment with pharmacotherapies has been limited to 12 weeks. However, an American Thoracic Society (ATS) systematic review of eight studies demonstrated that extended use of a controller therapy for greater than 12 weeks leads to significantly higher sustained quit rates and lower relapse rates compared with standard use of six to 12 weeks, with a number needed to treat (NNT) of 19 for one additional patient achieving abstinence at one year.9 The ATS recommends extended use of controller pharmacotherapies for up to one year for smoking cessation.9 A 2021 randomized controlled trial did not find improved abstinence with extended use of varenicline.16
Although medications have been traditionally restricted to individuals ready to quit, there is evidence that varenicline is effective for smoking cessation even in patients who are reluctant to quit.9 This indicates that varenicline, and possibly pharmacotherapy in general, plays an important role in disrupting the neurobiologic pathways that promote nicotine dependence and helps empower patients to quit. The ATS strongly recommends that physicians offer varenicline to patients with nicotine dependence even before they have expressed readiness to quit because this increases cessation at six months (NNT = 6).9
NICOTINE REPLACEMENT THERAPY
NRT is designed to reduce cravings and withdrawal symptoms by stabilizing the patient’s nicotine dose to approximate intake and then gradually reducing the dose, without the associated toxins found in cigarettes. The dose needed to mitigate symptoms can be estimated based on the patient’s average cigarette use. One cigarette contains approximately 2 mg of nicotine. The transdermal patch is the only long-acting NRT; the gum, lozenge, inhaler, and nasal spray are short-acting.2
Although smokers traditionally start NRT on their quit date, some evidence supports increased abstinence rates if NRT is started before the quit date.4,9 Patients taking any form of NRT are 55% more likely to sustain smoking cessation for at least six months (NNT = 17) compared with placebo or no NRT.17 Using a combination of long- and short-acting NRTs increases the quit rate by 25% over a single form and is recommended for all nonpregnant adult smokers, especially heavy smokers.6,13,18
Common adverse effects of NRT include headache, nausea, vomiting, indigestion, and sleep disturbance. NRT dependence is uncommon and outweighed by the overall benefits of smoking cessation.5 Side effect profiles by type of NRT are detailed in Table 3.8,14,15 Some NRTs are available over the counter, and some are prescription only.
BUPROPION SUSTAINED-RELEASE
Bupropion was the first non–nicotine-based pharmacotherapy approved for smoking cessation. Initially designed and marketed as an antidepressant, bupropion acts by weakly inhibiting the reuptake of norepinephrine and dopamine and partially blocking nicotine receptors, which reduce nicotine cravings.2,4,14,15,19 Bupropion is associated with a 64% higher likelihood of sustained smoking cessation (NNT = 14) compared with placebo or no pharmacotherapy, which is similar to the effectiveness of NRT.4,19 There is insufficient evidence to indicate whether the combination of bupropion and NRT is more effective than NRT alone.19
VARENICLINE
Varenicline is a partial agonist of the alpha4-beta2 nicotine receptor that reduces cravings and withdrawal symptoms and blocks the rewarding effect of nicotine.2,4,9 Patients taking varenicline are 2.2 times more likely to achieve sustained smoking cessation compared with placebo.13,20 Varenicline is the most effective FDA-approved pharmacotherapy for smoking cessation, with an NNT of 7 for abstinence at six months compared with bupropion and NRT.6,9,13,19,20
The ATS strongly recommends varenicline over bupropion and NRT.9 The ATS also conditionally recommends using varenicline plus a nicotine patch over varenicline alone, noting a 36% increase in sustained cessation rates and an NNT of 10 for six-month abstinence compared with varenicline alone. However, a 2021 randomized controlled trial did not show any difference between combining the nicotine patch with varenicline and using varenicline alone.16 There are no studies evaluating the combination of varenicline and short-acting NRTs.9 Evidence is insufficient to recommend the combination of bupropion and varenicline.19
OFF-LABEL USE OF PHARMACOTHERAPIES
Cytisine (Tabex) is a nicotine partial agonist that is licensed for use only in a few countries in Central and Eastern Europe. Two small trials suggest that cytisine leads to improved long-term abstinence without significant adverse events, although the evidence is low quality.20 Nortriptyline (Pamelor) titrated up to 75 to 100 mg per day and then taken for 12 weeks doubles the chance of quitting compared with placebo but may not be beneficial when added to NRT.21 Clonidine is less effective for smoking cessation than NRT, bupropion, and varenicline, and is associated with a dose-related increase in adverse events.21
Nonpharmacologic Interventions
Table 4 lists nonpharmacologic interventions for smoking cessation.22–40 The choice of interventions should be based on shared decision-making that includes patient preference and experience with previous quit attempts. Combining effective behavior interventions with pharmacotherapy improves cessation rates.13
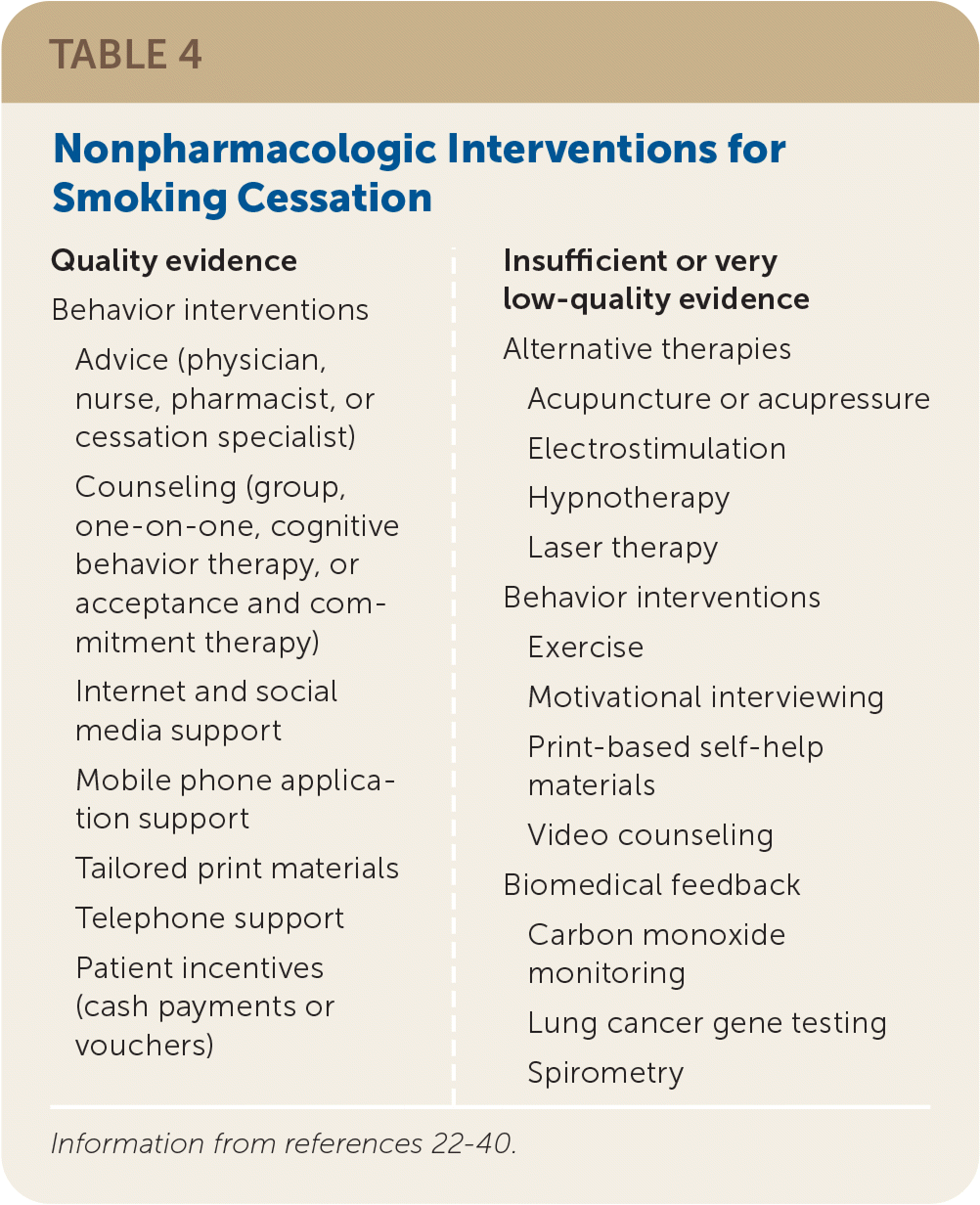
| Quality evidence Behavior interventions Advice (physician, nurse, pharmacist, or cessation specialist) Counseling (group, one-on-one, cognitive behavior therapy, or acceptance and commitment therapy) Internet and social media support Mobile phone application support Tailored print materials Telephone support Patient incentives (cash payments or vouchers) | Insufficient or very low-quality evidence Alternative therapies Acupuncture or acupressure Electrostimulation Hypnotherapy Laser therapy Behavior interventions Exercise Motivational interviewing Print-based self-help materials Video counseling Biomedical feedback Carbon monoxide monitoring Lung cancer gene testing Spirometry |
e-Cigarettes
e-Cigarettes are not FDA-approved tobacco cessation aids. However, people who want to quit smoking often use them.41,42 Pooled data from multiple studies showed that the use of e-cigarettes containing nicotine increases smoking cessation at six months (NNT = 25) compared with both NRT and e-cigarettes without nicotine.43 In contrast, a 2022 observational cohort study found that e-cigarette use results in a higher rate of smoking at 12 months (number needed to harm = 13).44 Switching from cigarette to e-cigarette dependency is a concern; continued use of e-cigarettes was as high as 80% in one study.43 In children and adolescents, the use of e-cigarettes increases probability, frequency, and intensity of combustible tobacco use.45 The USPSTF does not recommend for or against the use of e-cigarettes for smoking cessation, citing insufficient evidence and the need for ongoing studies.6
Common adverse effects of e-cigarettes include throat irritation, coughing, nausea, and sleep disruption; adverse effects and serious adverse events do not differ between nicotine and non-nicotine e-cigarettes.43 In 2019, an outbreak of lung injury associated with vaping emerged, with chest pain, cough, shortness of breath, gastrointestinal symptoms, fever, and chills. These lung injuries were likely caused by vitamin E acetate and other additives, mostly found in e-cigarettes containing tetrahydrocannabinol. Since 2019, cases of vaping-associated lung injury have significantly declined secondary to increased public awareness and the removal of vitamin E acetate from most products.46
Special Populations
PREGNANCY
All pregnant patients should be screened for tobacco use, advised to quit, and provided behavior interventions to help with cessation.6,47 Repeat screening may be useful, with recent studies showing a significant discordance between reported smoking and biomarkers to assess smoking.48 Smoking increases rates of ectopic pregnancy, low birth weight, preterm birth, miscarriage, placental abruption, and orofacial clefts.2,49 Smoking cessation during the first 15 weeks of pregnancy reduces the risk of low-birth-weight infants to that of never smokers.47 Pregnant people with fewer resources, lower education levels, and no support at home are least likely to quit smoking, suggesting that more resources are needed to help pregnant patients succeed.50
Behavior therapies are first-line treatment for smoking cessation in pregnant patients because of the uncertainty about the benefits and risks of pharmacotherapy in this population. There have been limited studies on the use of NRT for smoking cessation in pregnancy, and the USPSTF has concluded that evidence is insufficient to make a recommendation.6 A meta-analysis of five placebo-controlled trials demonstrated that NRT did not significantly improve smoking cessation in pregnant patients.6,13 The benefits and risks of NRT in pregnant people should be balanced against the risk of continued tobacco use.6
ADOLESCENTS
Adolescence is the primary time when people start smoking, and experimentation can turn to dependence. The USPSTF and AAFP recommend interventions to prevent the initiation of smoking in school-aged children and adolescents.51,52 Behavior interventions in the form of print materials or telephone, internet-based, or interactive computer support decrease smoking initiation in adolescents but do not increase smoking cessation.51 Trials assessing behavior interventions for smoking cessation in adolescents are limited by a low number of participants. Pharmacologic interventions are not recommended because of insufficient evidence in this population.51
MENTAL HEALTH
Patients with mental health disorders are twice as likely to smoke. The more severe the psychiatric diagnosis, the higher the prevalence of smoking. Moreover, smokers with mental illness smoke more heavily than other smokers.9
Lung Cancer Screening
The USPSTF recommends annual lung cancer screening with low-dose computed tomography for adults 50 to 80 years of age who have a 20-pack-year smoking history and currently smoke or have quit within the past 15 years.53 Lung cancer screening alone may not affect smoking cessation, regardless of the result. Therefore, smokers should be provided smoking cessation counseling at each lung cancer screening visit.54–56 The Centers for Medicare and Medicaid Services requires that clinicians provide counseling on abstinence maintenance or smoking cessation and appropriate cessation tools for screening reimbursement.57 A study demonstrated that the lung cancer screening guideline increases the likelihood of patients receiving smoking cessation interventions, even for smokers not eligible for screening.58 Despite this improvement, many clinicians and patients report a lack of smoking cessation discussions.59
Data Sources: A PubMed search was completed in clinical queries using the key terms smoking, tobacco, cessation, treatment, therapy, counseling, and medications. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. Also searched were the Agency for Healthcare Research and Quality Effective Healthcare Reports, the Centers for Disease Control and Prevention website, DynaMed, and Essential Evidence Plus. Search dates: February 8, 2022, and August 24, 2022.
This article updates previous articles on this topic by Larzelere and Williams8; Mallin60; and Okuyemi, et al.61
The contents of this article are solely the views of the authors and do not necessarily represent the official views of the U.S. Air Force, the U.S. military at large, the U.S. Department of Defense, or the U.S. government.
